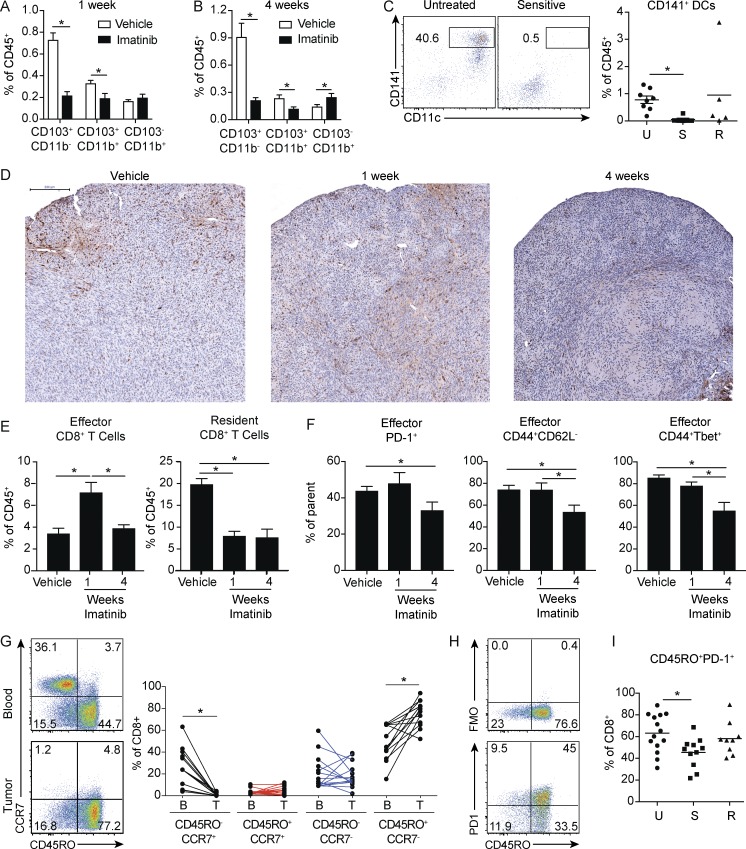
Figure 3.
Short- and long-term imatinib have divergent effects on antitumor effector CD8+ T cells. (A) Frequency of DC subsets in tumors of KitV558Δ/+ mice treated with vehicle or imatinib for 1 wk (four mice/group). (B) Frequency of DC subsets in tumors of KitV558Δ/+ mice treated with vehicle or imatinib for 4 wk (four mice/group). A and B are representative of two independent experiments. (C) Human GIST specimens characterized by sensitivity to imatinib treatment (19 specimens from 14 patients) were analyzed for CD141+ DCs. Untreated tumors (U) were from patients who never received a tyrosine kinase inhibitor before surgery. Sensitive tumors (S) were responding to imatinib or another tyrosine kinase inhibitor at the time of surgery. Resistant tumors (R) were progressing despite treatment with imatinib or another tyrosine kinase inhibitor. (D) KitV558Δ/+ mice were treated with vehicle or imatinib for 1 or 4 wk and stained for CD8. Bar represents 200 µm. Representative of three mice/group. (E) Frequency of effector CD8+CD103− and resident CD8+CD103+ T cells from tumors of KitV558Δ/+ mice treated with vehicle or imatinib for 1 or 4 wk (four mice/group). (F) Frequency of PD-1+, CD44+CD62L−, and CD44+ Tbet+ effector CD8+CD103− T cells from tumors of KitV558Δ/+ mice treated with vehicle or imatinib for 1 or 4 wk (four mice/group). E and F represent two pooled experiments. (G) Matched peripheral blood and untreated human GIST specimens (14 tumor specimens from 10 patients) were analyzed for frequency of CD8+ T cell memory subsets. (H) Representative flow cytometry of CD45RO and fluorescence minus one (FMO) or PD-1 in CD8+ T cells from untreated human GIST specimens. (I) 38 human GIST specimens from 28 patients were characterized by sensitivity to imatinib treatment and analyzed for CD8+CD45RO+PD1+ T cells. Data represent mean ± SEM; P values were calculated using a Student’s t test; *, P < 0.05.