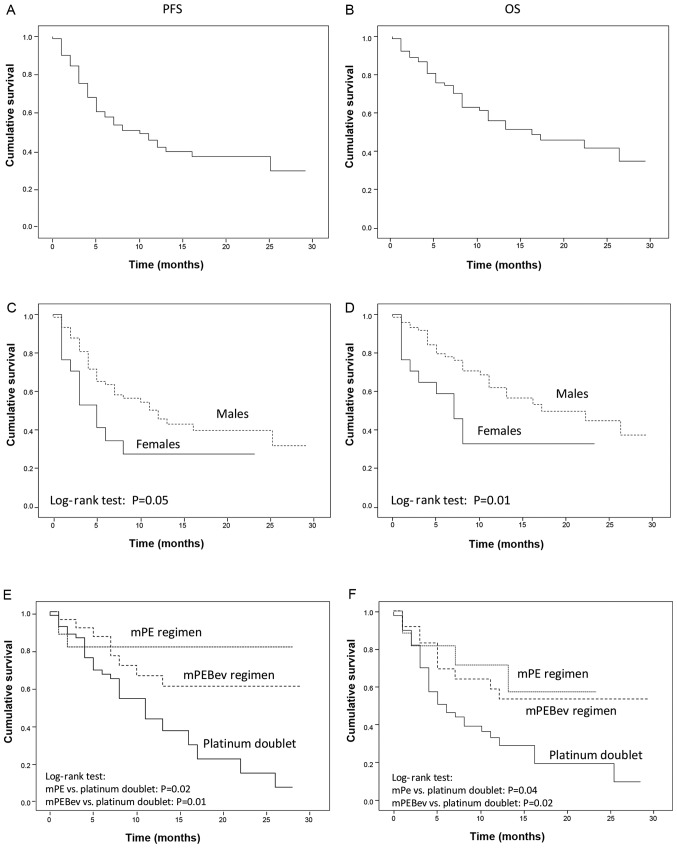
Figure 1.
PFS and OS recorded in mNSCLC patients receiving Nivolumab as salvage therapy (a Log-rank test). (A and B) Results recorded on the overall population. (C and D) Results in comparison between male and female sex. (E and F) Results recorded in patients that had undergone standard chemotherapy, mPE regimen or mPEBev regimen prior the immune-oncological treatment with nivolumab. PFS, progression free survival; OS, overall survival; mNSCLC, metastatic non-small cell lung cancer; mPE, fractioned cisplatin (30 mg/m2 days 1-3q21) and metronomic oral etoposide (50 mg days 1-15q21); mPEBEV, fractioned cisplatin (30 mg/m2 days 1-3q21) and metronomic oral etoposide (50 mg days 1-15q21) in combination with bevacizumab (5 mg/kg day 3q21).