Figure S3.
Initial cryo-EM Analysis of the Pan2-Pan3-90A RNP Complex, Related to Figure 3
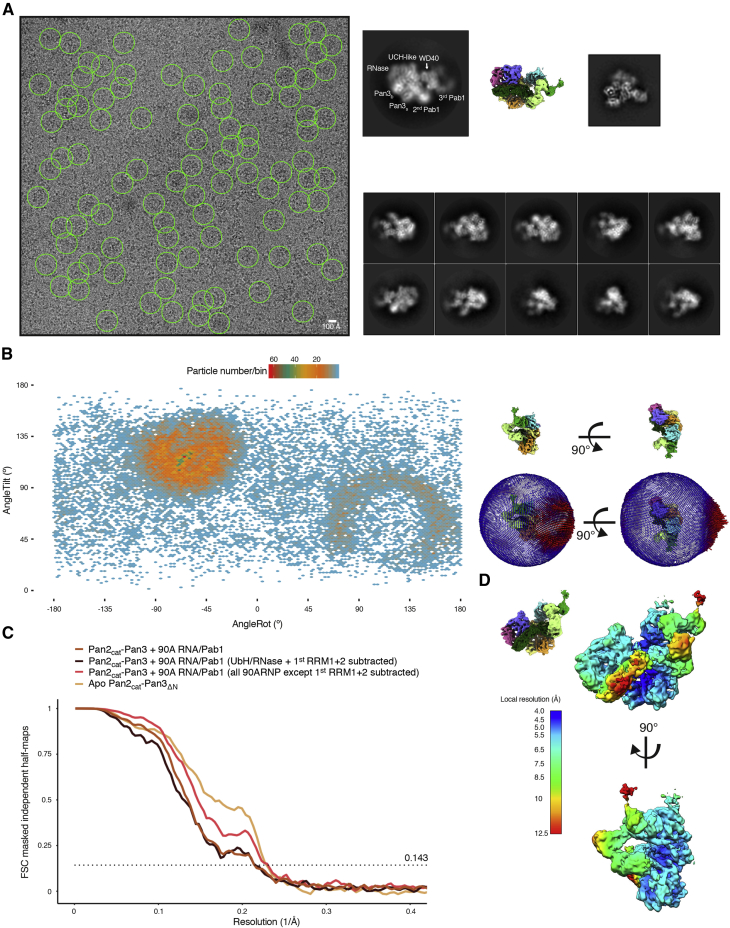
(A) Representative micrograph (at 0° pre-tilt, on the left-hand side) and reference free 2D class averages (on the right) of the Pan2-Pan3-90A RNP. The scale-bar in the cryo-EM micrograph corresponds to 100 Å and the green circles (260 Å diameter) indicate particles contributing to the initial reconstruction with a nominal global resolution of 7.1 Å (see Figure S4). The 2D class average at the top on the right is contrasted with the 3D reconstruction of the Pan2-Pan3-90A RNP complex and a 2D class average of the Pan2-Pan3 apo data in similar orientations.
(B) Angular distribution of particles contributing to the full Pan2-Pan3-90A RNP complex reconstruction. In the panel on the left tilt and rotation angles were plotted against one another for the final 4.8 Å 3D reconstruction (Map 1 in Figure S4). The color of each sampling bin indicates the number of particles in the respective bin. As in the spherical angular distribution representation on the right, bins colored in blue have fewer particles and red ones more (29 165 particles in total).
(C) Fourier Shell Correlation (FSC) of masked independent half-maps of the Pan2-Pan3–90A RNP reconstructions used for modeling and structure interpretation (see also Figure S4 for details). According to the gold standard FSC cut off of 0.143 (Rosenthal and Henderson, 2003) the nominal overall resolution of the full Pan2-Pan3 reconstruction is 4.8 Å (Map 1, light brown curve). The reconstruction focused on the RNase/RRM1-RRM2 interaction has a nominal global resolution estimated as 4.5 Å (Map 3, red curve) and that of the reconstruction focusing on the recognition of the Pab1-Pab1 oligomerization interface is 4.7 Å (Map 2, dark brown curve). The FSC of the apo Pan2-Pan3 is also blotted for comparison (sand colored curve).
(D) Map of the full Pan2-Pan3–90A RNP colored according to local resolution estimation. The core of Pan2-Pan3 and the Pan2-Pan3–90A/Pab1 interacting regions are resolved at higher resolution whereas parts of the 90A/Pab1 RNP not in contact with the deadenylase complex are less well resolved.