Figure S6.
Determinants of Pan2-Pan3 Recruitment to the Poly(A)/RNP, Related to Figure 5
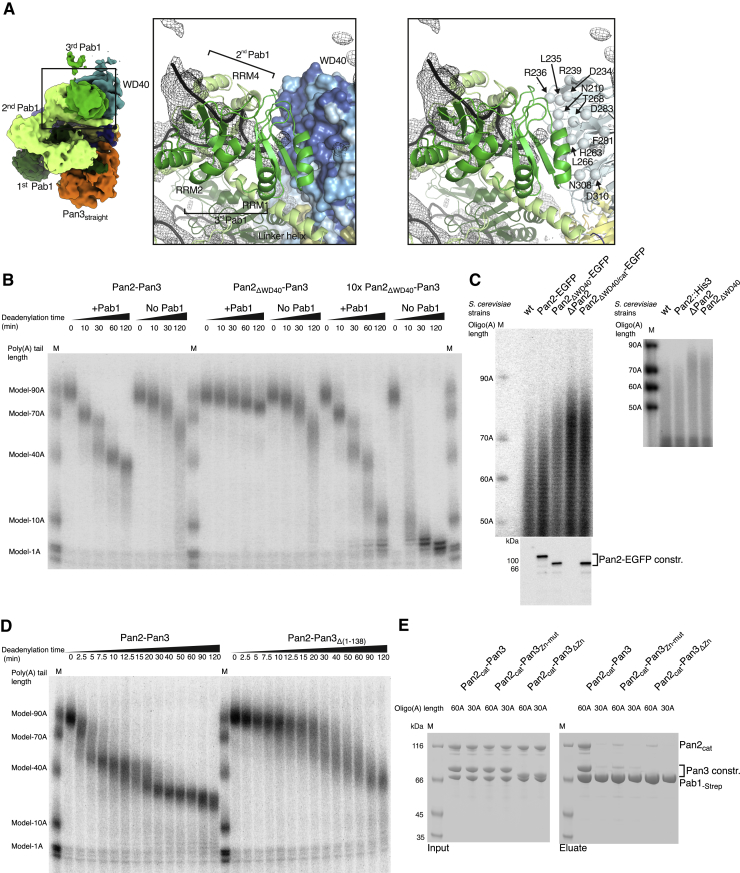
(A) Details of the interactions between the second Pab1-Pab1 oligomerization interface of the 90A RNP and the Pan2 WD40 domain. The panel on the left highlights the overall position of the interface in the context of the reconstruction (shown as segmented density, as in Figure 3B). Conserved surface residues of the WD40 domain in proximity to the Pab1-Pab1 interface are accentuated in a dark blue shade in the surface representation (panel in the middle) and as spheres in the cartoon model on the right.
(B) Pan2ΔWD40-Pan3 deadenylation activity is not stimulated by Pab1. 5′ radioactively labeled model-90A RNA was mixed with Pab1 (in a 1:3 RNA:protein ratio,”+Pab1”) and incubated with either wild-type Pan2-Pan3 (left hand side of the gel) or Pan2ΔWD40-Pan3 (right hand side of the gel, equimolar and 10x the amount of wild-type Pan2-Pan3) over a 2 h period. The model-90A RNA in the absence of Pab1 (“no Pab1”) was in parallel also used as substrate in similar deadenylation reactions. At indicated time points samples were taken and analyzed on a 6% Urea-PAGE followed by phosphorimaging.
(C) The Pan2 WD40 domain influences poly(A) tail length in vivo. The upper panel shows phosphorimages of 8% UREA-PAGE of the pCp labeled, RNase A treated poly(A) isolations from mutant Pan2 yeast strains (Pan2-EGFP tagged strains on the left, S. cerevisiae strains carrying untagged Pan2 variants on the right). In the bottom panel is the respective anti-EGFP western blot.
(D) Removal of the Pan3 N terminus has a limited effect on deadenylation activity of a yeast 90A RNP. 5′ radioactively labeled model-90A RNA was mixed with Pab1 (in a 1:3 RNA:protein ratio) and incubated with either wild-type Pan2-Pan3 (left hand side of the gel) or Pan2-Pan3Δ (1–138) (right hand side of the gel) over a 2 h period. At indicated time points samples were taken and analyzed on a 6% Urea-PAGE followed by phosphorimaging.
(E) The Pan3 N-terminal Zn-fingers do not contribute to the poly(A) length dependency of the Pan2-Pan3–poly(A)/Pab1 interaction but increase affinity for the poly(A)/Pab1 RNP in co-precipitation experiments. Pan2cat-Pan3, Pan2cat-Pan3Zn-mut (in which the three Cys and one His coordinating the Zn ion have been mutated to Ser or Ala respectively) as well as Pan2cat-Pan3ΔZn (which is Pan2cat-Pan3Δ[1-74]) were preys in a Strep-Tactin pull-down with 30A/Pab1-Strep and 60A/Pab1-Strep as bait. The eluate off the Strep-Tactin resin was analysed on a 4%–12% SDS-PAGE followed by Coomassie staining.