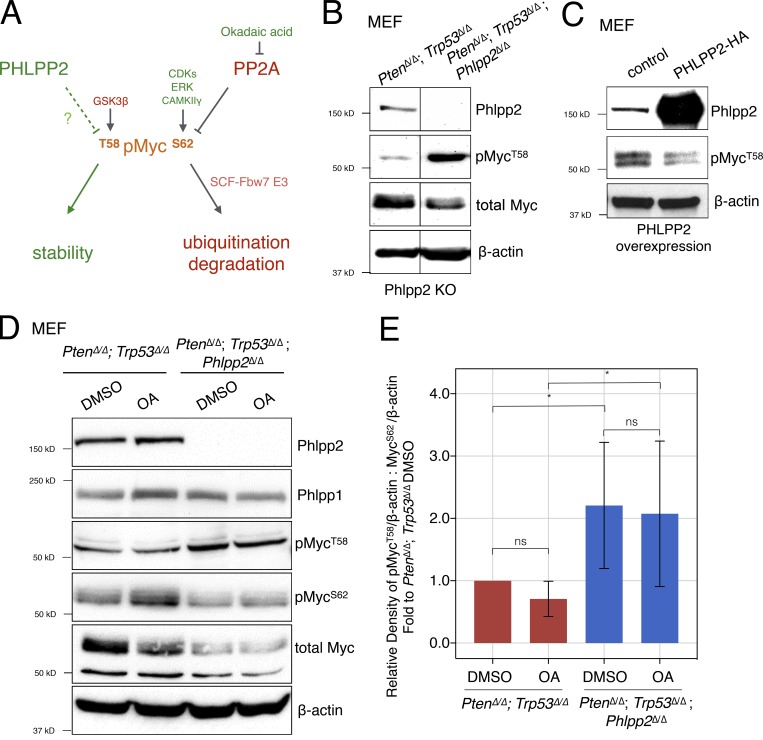
Figure 2.
Phlpp2 status controls phosphorylation of Myc. (A) Scheme representing regulators of MYC protein through T58 and S62 phosphorylation sites. These sites can be phosphorylated by GSK3β and CDK1/ERK/CAMKIIγ, respectively. Note that decreased ratios of T58 to S62 phosphorylation and T58 mutation to non–phospho-mimetic residues (e.g., T58A) are recurrent in human cancer. (B) Phlpp2 knockout in Pten/Trp53-null MEFs increases phosphorylation at the T58 site. Lines indicate splicing of three irrelevant lanes. (C) Overexpression of PHLPP2 leads to decreased T58 phosphorylation. (D) Treatment of Pten/Trp53-null MEFs with OA results in an increase in Myc phosphorylation on S62, but not T58. Total levels of Myc were reduced upon deletion of Phlpp2. (E) Densitometric analysis shows that the phosphorylation ratio of T58 to S62 is significantly increased in the triple-deleted MEFs. Data are presented as the densitometric measurements of pMycT58 and MycS62 normalized to β-actin and then these ratios of pMycT58 to MycS62 were used in graph as fold to PtenΔ/Δ;Trp53Δ/Δ DMSO. Error bars, SD; n = 5; one-way ANOVA, P values corrected for multicomparison testing using the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli, with a false discovery rate at 10%. *, P < 0.05. KO, knockout; ns, not significant.