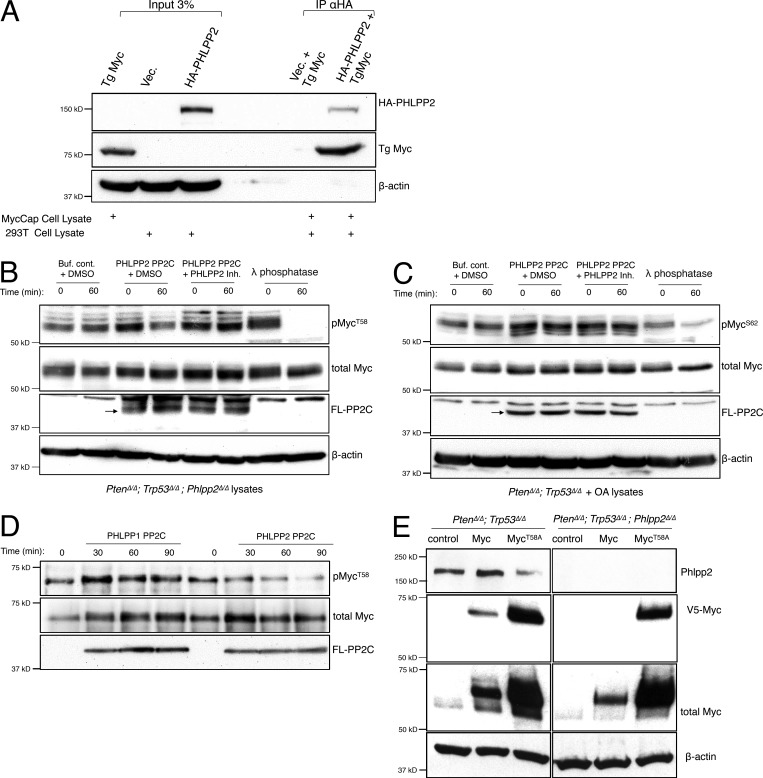
Figure 4.
Phlpp2 dephosphorylates Myc directly. (A) Co-immunoprecipitation experiments revealed that overexpressed HA-tagged PHLPP2 pulled-down transgenic (Tg) Myc (from MycCaP cells), consistent with a direct interaction. (B) PHLPP2 directly dephosphorylates T58 on Myc. Incubation with the FLAG-tagged PHLPP2 PP2C domain (arrow) resulted in a decrease in T58 phosphorylation after 60 min of incubation (lanes 3 and 4). Dephosphorylation was blocked by a small-molecule inhibitor of PHLPP2 (lanes 5 and 6). (C) PHLPP2 does not dephosphorylate S62 on Myc. Incubation with the FLAG-PP2C domain of PHLPP2 (arrow) did not affect S62 phosphorylation after 60 min of incubation (lanes 3 and 4). Dephosphorylation was not affected by a small-molecule inhibitor of PHLPP2 (lanes 5 and 6). (D) The purified FLAG-tagged PP2C domain of PHLPP2 was incubated with phosphorylated purified Myc protein for different time points. Incubation with the PP2C domain of PHLPP1 did not affect T58 even after 90 min. In contrast, incubation with the PP2C domain of PHLPP2 resulted in a decrease in T58 phosphorylation that was most significant after 90 min. (E) MycT58A levels increase compared with WT MYC regardless of Phlpp2 demonstrating that this site is key for Phlpp2-mediated Myc regulation. wt, wild-type.