Figure 1.
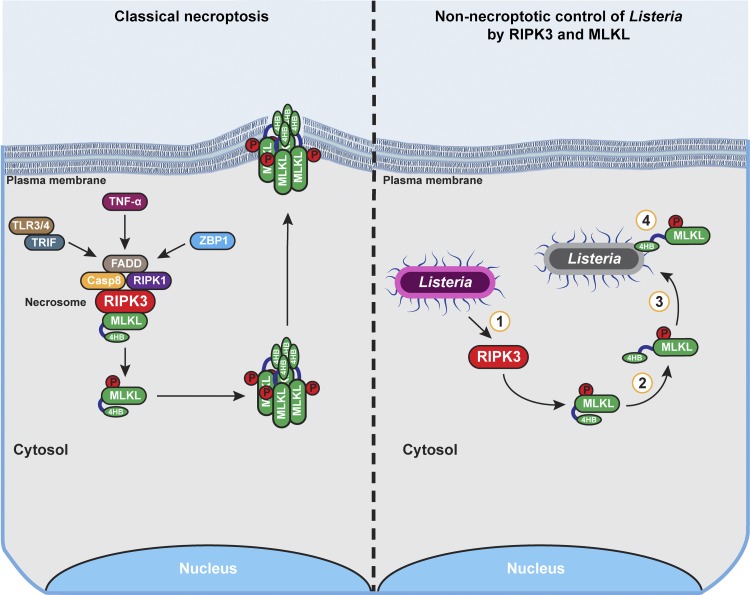
The necroptosis machinery promotes noncytolytic clearance of Listeria from epithelial cells. In classical necroptosis signaling (left), activation of RIPK3 by cytokines (e.g., TNF-α) or pattern recognition recptors (e.g., TLRs3/4 and ZBP1) results in formation of a RIPK3-containing necrosome in which MLKL is phosphorylated and activated. MLKL subsequently oligomerizes and undergoes conformational changes that expose its phospholipid binding 4HB domain. Active MLKL oligomers traffic to the plama membrane, where they form pores that eventually promote necrotic lysis of the cell. In contrast, during Listeria infection of epithelial cells (right), RIPK3 is activated and phosphorylates MLKL, but MLKL does not oligomerize and target the plasma membrane for disruption. Instead, phosphorylated MLKL directly associates with cytosolic Listeria and inhibits its replication. The key steps in this process that merit further investigation are depicted by numbers within circles: (1) the mechanism by which Listeria activates RIPK3, (2) the process governing activation of MLKL without inducing its oligomerization, (3) the means by which MLKL localizes to cytosolic Listeria, and (4) the mechanism by which MLKL limits Listeria replication in the cytosol.