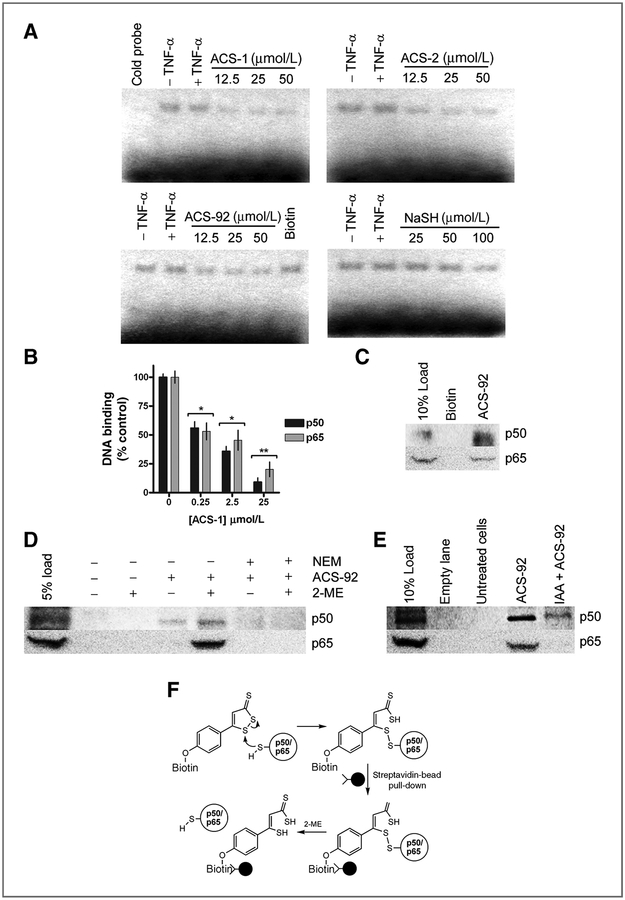
Figure 4.
Dithiolethiones covalently react with NF-kB subunits to inhibit DNA binding. A, images from electrophoresis mobility shift assay experiments from MDA-MB-468 cells treated for 2 hours with ACS-1 (with cold probe competition), ACS-2, ACS-92 (with biotin control), and NaSH before TNF-α stimulation for 3 hours. B, recombinant human p50 and p65 were incubated with ACS-1 and DNA binding was measured as described. Statistical differences versus control samples were calculated by one-way ANOVA with the Dunnett posttest (*, P < 0.05; **, P < 0.01). C, p50 and p65 protein pull down from MDA-MB-231 whole-cell lysate by biotin or ACS-92 compared with lysate load control. D, Western blot of p50 and p65 eluted from ACS-92 pull down of MDA-MB-231 whole-cell lysate in the presence or absence of 2-ME. E, serum-starved MDA-MB-231 treated with or without IAA (100 μmol/L) before ACS-92 (50 μmol/L). Whole-cell lysates were incubated with streptavidin beads, and p50 and p65 were eluted in the presence of 2-ME and measured by Western blot. F, schematic representing ACS-92 covalently reacting with p50 and p65, which are pulled down from cell lysate via streptavidin-conjugated beads. Protein is eluted from the beads only in the presence of thiol-reducing agent (2-ME), indicating a disulfide bond formed between ACS-92 and the NF-κB subunits.