Figure 2.
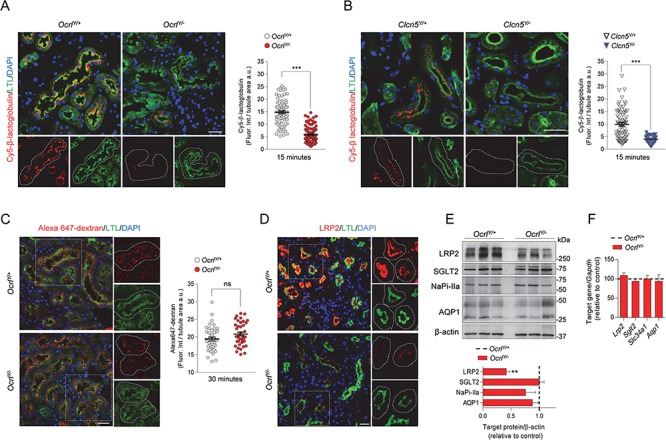
Defective receptor-mediated endocytosis in Dent disease mice. (A,B) Representative confocal micrographs showing Cy5-labeled β-lactoglobulin (red, 1 mg/kg B.W.) or (C) Alexa 647-labeled dextran uptake (red, 6 mg/kg B.W.) after 15 and 30 min from tail vein injections, respectively, and quantifications of the corresponding fluorescent signal in LTL+ (Lotus tetragonolobus Lectin, green) PTs from Ocrl and Clcn5 mouse kidneys (n = 90 Ocrl and n = 70 Clcn5 PTs for Cy5-labeled β-lactoglobulin uptake; n = 40 Ocrl PTs for Alexa 647 dextran uptake) n = 2 per group. Each dot represents fluorescence intensity in one PT; fluorescence intensity was normalized on tubule area; two-tailed unpaired Student’s t-test, ***P < 0.001 relative to OcrlY/+or Clcn5Y/+ kidneys. ns: not significant. Insets: high magnification of Cy5-labeled β-lactoglobulin+ or Alexa 647 dextran+ structures in LTL+ PTs. (D) Representative confocal micrographs showing LRP2 (red) expression in LTL+ (green) PTs of Ocrl mouse kidneys. Insets: high magnification of LRP2+ structures in LTL+ PTs. (E) Western blotting and densitometry analyses of LRP2, SGLT2, NaPi-IIa and AQP1 protein levels in whole-kidney lysates from Ocrl mice. β-actin was used as loading control. Protein levels normalized on β-actin and relative to OcrlY/+ mice (black dotted line; LRP2, n = 6 mice per group; SGLT2, NaPiIIa and AQP1, n = 3 mice per group; Mann–Whitney U test, **P < 0.01 relative to OcrlY/+ kidneys). (F) The mRNA kidney levels of Lrp2, Sglt2, Slc34a1 and Aqp1 were analyzed by real-time qPCR. Gene target expression normalized to Gapdh and relative to OcrlY/+ mice (black dotted line; n = 3 mice per group). Nuclei counterstained with DAPI (blue) in (A–D). Scale bars: 25 μm. Plotted data represent mean ± SEM.
