Figure 2.
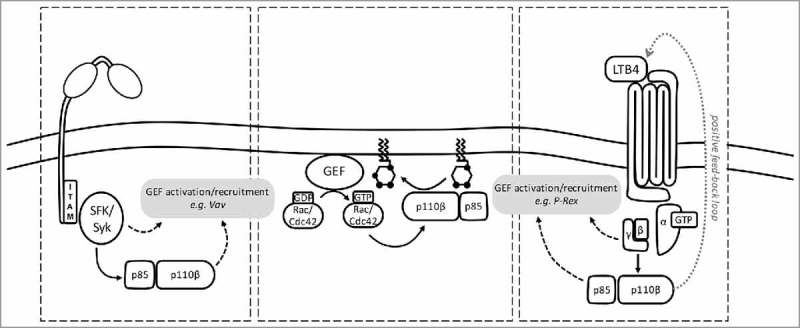
PI3Kβ is activated by phosphopeptide, Gβγ and Rac/Cdc42 in the integrin/immune complex-stimulated neutrophil. (Left) Neutrophil integrin or FcγR ligation causes Src family kinase (SFK)-dependent phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs), triggering the activation of Syk kinase, which in turn recruits the p85 adaptor in an SH2 domain- and phosphotyrosine motif-dependent fashion to activate PI3Kβ. Integrin/FcγR ligation and the PIP3 also activate Rac/Cdc42 GEFs. (Right) In a paracrine feed-forward loop, PI3Kβ drives LTB4 production. LTB4 triggers activation of its GPCR (BLT1), resulting in release of Gβγ subunits activate PI3Kβ by binding to p110β. GPCR ligation and PIP3 also activate Rac/Cdc42 GEFs. (Center panel) Rac/Cdc42 GEFs enable GTP-loading of Rac/Cdc42, which can then bind the p110β RBD, to further activate PI3Kβ. Full activation of PI3Kβ requires phosphotyrosine motifs, Gβγ and Rac/Cdc42.
