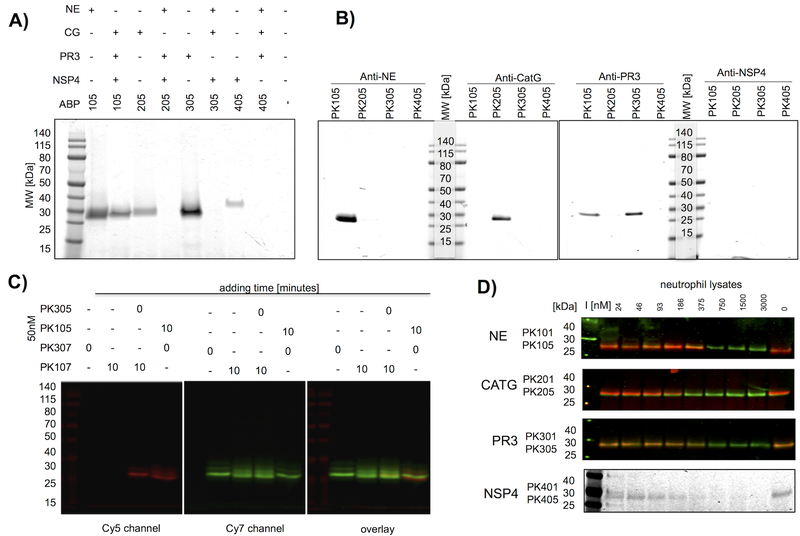
Figure 3. Selectivity of champion NSP probes.
(a) Champion probes (100nM) were incubated with their target NSP or with a mixture of the other NSPs for 10 minutes followed by SDS PAGE and fluorescence imaging of the gel. (b) Neutrophil extracts were treated with probes (100nM), followed by a protease inhibitor cocktail (1mM 1,10-phenanthroline, 50μM DCI, 10μM E-6) and the indicated specific antibodies, captured by Protein A/G-Plus agarose beads, followed by SDS PAGE of the captured proteins, and fluorescence imaging of the gels. No bands were detected with the NSP4 antibody capture, likely because the antibody is poor at recognizing the native protein. (c) PK10X series probes preferentially label NE in the presence of PR3. PR3 probes were added to neutrophil extracts at time = 0, followed in lanes 3–4 by a second NE probe after 10 min as indicated above the gel. Each sample was incubated for a total of 20 min before SDS page, and gels were analyzed for Cy5 and Cy7 fluorescence. All probes were used at 50nM final concentration. PK107 labels NE glycoforms and a small amount of PR3, whereas PK305 labels PR3 exclusively. The small amount of cross reactivity of PK10X series probes (see the faint overlaid band in lane 3) represents off target labeling of PR3 by PK107. Images describe the individual channels that form the basis of the overlay. (d) Lysates were treated with PKX01 (biotin-tagged) activity-based probes to act as titration inhibitors (I) in the indicated concentration range for 45 minutes at 37°C, followed by 200nM Cy5 probes (PK X05 series) and an additional 30 minutes incubation. For NE, CATG and PR3, western blots were performed and membranes were stained with appropriate antibodies. The membranes were imaged for the Cy5 probes (red) and IRDye800W® (green).