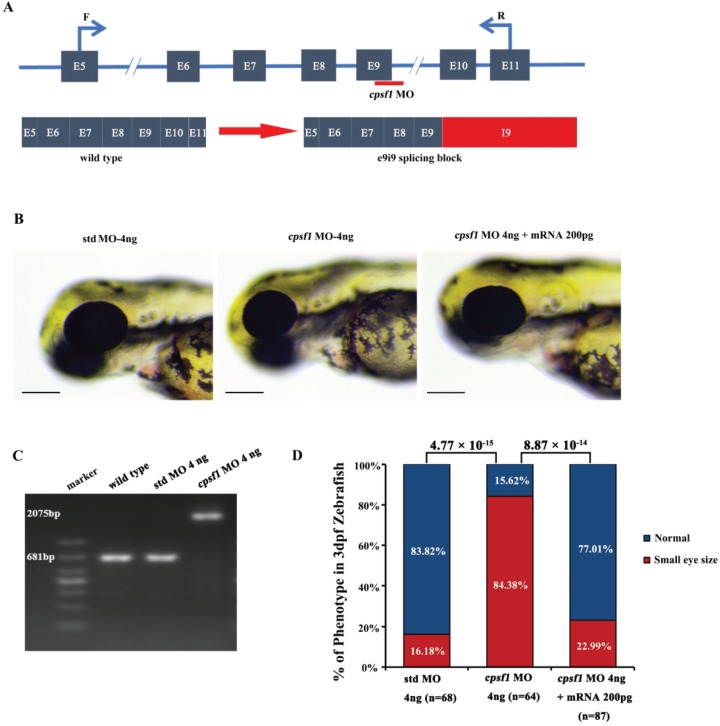
Figure 4.
Knockdown of cpsf1 in zebrafish caused abnormal ocular morphogenesis. (A) Diagram illustrating the structure of exons 5 to 11 of the zebrafish cpsf1 gene, the cpsf1 MO targeting sites, and the primers used to test splicing products before and after cpsf1 MO microinjection. (B) Eye size in zebrafish at 3 dpf. Left: microinjection of std MO (4 ng), middle: microinjection of cpsf1 MO (4 ng), right: co-injection of cpsf1 MO (4 ng) and cpsf1-mRNA (200 pg). The eye diameter (eye size) was 294.09 ± 10.28 μm (n = 68) for the std control, 263.55 ± 20.85 μm (n = 64) for the cpsf1 morphants and 289.97 ± 15.47 μm (n = 87) for the zebrafish subjected to cpsf1-MO/cpsf1-mRNA co-injection. Small eye size was detected in cpsf1 morphants, but this phenotype could be rescued by 200 pg of cpsf1-mRNA. Std MO-injected zebrafish were used as a control in this study. (C) The efficiency of cpsf1 MO knockdown was tested by RT-PCR using the primers shown in (A). The amplicons from WT and std MO-injected zebrafish RNA were 681 bp, while cpsf1 MO disrupted the splicing of e9i9, resulting in the occurrence of a larger amplicon of 2075 bp at 24 hours post fertilization (hpf). (D) Quantification of small eye size proportions in zebrafish at 3 dpf. The data showed that the proportion of small eye size was significantly increased in cpsf1 morphants compared to that of the std control (P = 4.77 × 10−15), and the small eye size phenotype could be rescued by cpsf1-mRNA (P = 8.87 × 10−14) based on the Chi-square test (P < 0.017, or 0.05/3, was considered as statistically significant). The number of zebrafish injected in each group is indicated under each column.