Figure 1.
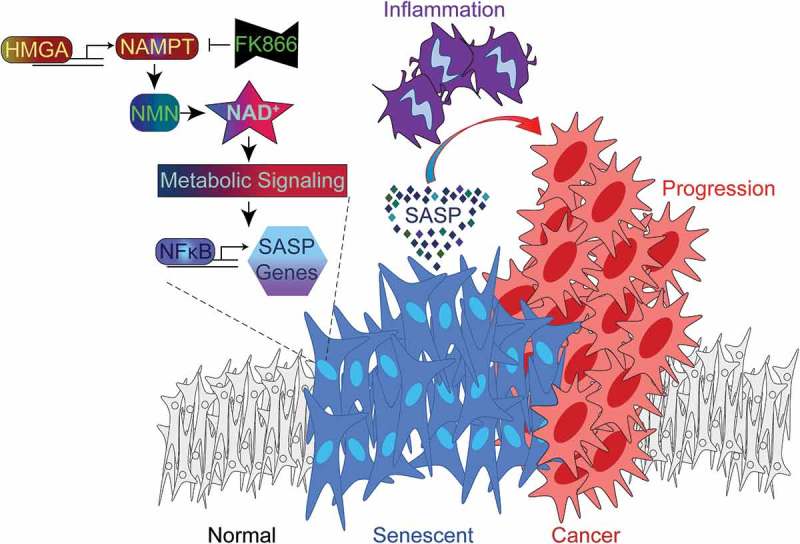
HMGA-NAMPT-NAD+ signaling promotes a pro-inflammatory SASP and drives cancer progression. During cellular senescence, high mobility group A (HMGA) proteins upregulate nicotinamide phosphoribosyltransferase (NAMPT) expression and stimulate nicotinamide adenine dinucleotide (NAD+) metabolism. This metabolic change suppresses AMP-activated protein kinase-p53 signaling that would serve to restrain nuclear factor-kappa B (NFkB) activity and the pro-inflammatory senescence-associated secretory phenotype (SASP). As a result, NAD+ metabolism stimulates the SASP of senescent cells, which can be inhibited by FK866 treatment or exacerbated by nicotinamide mononucleotide (NMN) treatment. In the pancreatic ductal adenocarcinoma (PDAC) mouse model, pancreas-specific oncogenic K-Ras expression induces pancreatic intraepithelial neoplasia (PanIN) lesions that are enriched in senescent cells. The PanIN lesions create a pro-inflammatory microenvironment that drives PDAC progression. PDAC progression is enhanced by exacerbating the SASP through stimulation of NAD+ metabolism using NMN treatment.
