Abstract
Background
The main complication of cerebrospinal fluid (CSF) shunt surgery is shunt infection. Prevention of these shunt infections consists of the perioperative use of antibiotics that can be administered in five different ways: orally; intravenously; intrathecally; topically; and via the implantation of antibiotic‐impregnated shunt catheters.
Objectives
To determine the effect of different routes of antibiotic prophylaxis (i.e. oral, intravenous, intrathecal, topical and via antibiotic‐impregnated shunt catheters) on CSF‐shunt infections in persons treated for hydrocephalus using internalised CSF shunts.
Search methods
We conducted a systematic electronic search without restrictions on language, date or publication type. We performed the search on the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE and Embase, with the help of the Information Specialist of the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group. The search was performed in January 2018.
Selection criteria
All randomised and quasi‐randomised controlled trials that studied the effect of antibiotic prophylaxis, in any dose or administration route, for the prevention of CSF‐shunt infection in patients that were treated with an internal cerebrospinal fluid shunt. Patients with external shunts were not eligible.
Data collection and analysis
Two review authors independently extracted data from included studies. We resolved disagreements by discussion or by referral to an independent researcher within our department when necessary. Analyses were also performed by at least two authors.
Main results
We included a total of 11 small randomised controlled trials, containing 1109 participants, in this systematic review. Three of these studies included solely children, and the remaining eight included participants of all ages. Most studies were limited to the evaluation of ventriculoperitoneal shunts. However, five studies included participants with ventriculoatrial shunts, of which one study contained four participants with a subduroperitoneal shunt. We judged four out of 11 (36%) trials at unclear risk of bias, while the remaining seven trials (64%) scored high risk of bias in one or more of the components assessed.
We analysed all included studies in order to estimate the effect of antibiotic prophylaxis on the proportion of shunt infections regardless of administration route. Although the quality of evidence in these studies was low, there may be a positive effect of antibiotic prophylaxis on the number of participants who had shunt infections (RR 0.55, 95% CI 0.36 to 0.84), meaning a 55% reduction in the number of participants who had shunt infection compared with standard care or placebo.
Within the different administration routes, only within intravenous administration of antibiotic prophylaxis there may be evidence of an effect on the risk of shunt infections (RR 0.55, 95% CI 0.33 to 0.90). However, this was the only route that contained more than two studies (8 studies; 797 participants). Evidence was uncertain for both, intrathecal administration of antibiotics (RR 0.73, 95% CI 0.28 to 1.93, 2 studies; 797 participants; low quality evidence) and antibiotic impregnated catheters (RR 0.36, 95% CI 0.10 to 1.24, 1 study; 110 participants; very low quality evidence)
Authors' conclusions
Antibiotic prophylaxis may have a positive effect on lowering the number of participants who had shunt infections. However, the quality of included studies was low and the effect is not consistent within the different routes of administration that have been analysed. It is therefore uncertain whether prevention of shunt infection varies by different antibiotic agents, different administration routes, timing and doses; or by characteristics of patients, e.g. children and adults. The results of the review should be seen as hypothesis‐generating rather than definitive, and the results should be confirmed in adequately powered trials or large multicentre studies in order to obtain high‐quality evidence in the field of ventricular shunt infection prevention.
Plain language summary
Route of antibiotic prophylaxis for prevention of cerebrospinal fluid‐shunt infection
Review question
We reviewed evidence about the effect of different administration routes of antibiotics given to prevent shunt infection in people who received a cerebrospinal fluid shunt.
Background
People with hydrocephalus (an excessive amount of cerebrospinal fluid within the brain, due to a blockage within their brain cavities or in their reabsorbing system) can be treated by implanting a cerebrospinal fluid shunt. This shunt is a tube running from the brain to either the heart or the abdomen in order to drain the excessive amount of cerebrospinal fluid to other body compartments where it will be reabsorbed. One of the most common problems after the implantation is infection of the shunt. Patients become sick and in most cases the shunt needs to be removed and a new one needs to be implanted after the patient has recovered. In order to reduce the number of infections surgeons administer antibiotics before, during or after surgery, or in various combinations, in order to protect the patient against bacteria that can infect the shunt. These antibiotics can be administered in different ways: orally; intravenously; directly into the brain cavities; directly on the shunt; and via the implantation of antibiotic‐impregnated shunt catheters.
Results
We included 11 studies up to January 2018 in this review, comprising a total of 1109 participants who had received a cerebrospinal fluid shunt for hydrocephalus. The majority of the included studies were small and of variable duration (from eight weeks to more than one year). We found that administration of antibiotics is effective in the prevention of shunt infections (very low quality evidence). As the included studies are few, the interventions used differed markedly and the certainty of the evidence for our outcomes was very low, our results prevented a clear conclusion as to what type of antibiotic and administration route is most effective in the prevention of shunt infection.
Summary of findings
Summary of findings for the main comparison. Antibiotic prophylaxis versus no antibiotic prophylaxis / standard care for CSF‐shunt infections.
| Antibiotic prophylaxis versus no antibiotic prophylaxis / standard care for CSF‐shunt infections | ||||||
| Patient or population: CSF‐shunt infections Setting: Hospital Intervention: antibiotic prophylaxis Comparison: no antibiotic prophylaxis / standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no antibiotic prophylaxis / standard care | Risk with antibiotic prophylaxis | |||||
| Number of participants experiencing shunt infection after administration of antibiotic prophylaxis follow up: mean 6.4 months | Study population | RR 0.55 (0.36 to 0.84) | 1109 (11 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 118 per 1.000 | 65 per 1.000 (43 to 99) | |||||
| Number of participants experiencing shunt infection after administration of intravenous antibiotic prophylaxis follow up: mean 5.5 months | Study population | RR 0.55 (0.33 to 0.90) | 797 (8 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | ||
| 116 per 1.000 | 64 per 1.000 (38 to 104) | |||||
| Number of participants experiencing shunt infection after administration of intrathecal antibiotic prophylaxis follow up: mean 10 months | Study population | RR 0.73 (0.28 to 1.93) | 202 (2 RCTs) | ⊕⊕⊝⊝ LOW 5 | ||
| 100 per 1.000 | 73 per 1.000 (28 to 193) | |||||
| Number of participants experiencing shunt infection after implantation of antibiotic impregnated catheters follow up: median 9 months | Study population | RR 0.36 (0.10 to 1.24) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 6 7 | ||
| 167 per 1.000 | 60 per 1.000 (17 to 207) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to indirectness of evidence, since control groups differed a lot. For example, one study compared the used of antibiotic prophylaxis to current practice in a multicenter design.
2 Downgraded one level because four studies were considered at serious risk of bias at at least three forms of bias
3 Downgraded one level due to indirectness of evidence, since control groups differed a lot.
4 Downgraded one level because two studies were considered at serious risk of bias at at least three forms of bias
5 Downgraded two levels because both studies were considered at serious risk of bias at at least three forms of bias
6 Downgraded two levels because the study was considered at serious or unclear risk of bias at five out of seven items
7 Downgraded one level, since only one study was included in this subgroup and the total number of participants in this study was low
Background
Description of the condition
Hydrocephalus is a condition in which cerebrospinal fluid (CSF) accumulates in the cerebral ventricles and subarachnoid spaces, resulting in dilatation of the ventricular system and an increase in intracranial pressure (Rekate 1999). There are two basic forms of hydrocephalus: non‐communicating and communicating. Non‐communicating hydrocephalus is caused by structural blockage of the CSF within the ventricular system and is the most common form of hydrocephalus in children. Communicating hydrocephalus can be caused either by the excessive production of CSF by the plexus choroideus or by inadequate resorption of CSF by the subarachnoid villi. Treatment of hydrocephalus comprises the drainage of excessive CSF through the implantation of shunts (a tube to drain cerebrospinal fluid from the brain to a body cavity, usually to the abdominal cavity), external drains (a tube to drain cerebrospinal fluid out of the body) or an endoscopic third ventriculostomy (a procedure in which cerebrospinal fluid circulation is restored by making a passage through the floor of the third ventricle). The choice of treatment and its efficacy differs according to the individual's age and the aetiology of the condition (Fu 2002; Hebb 2001; Limbrick 2014). The main complication of CSF‐shunt surgery is the incidence of CSF‐shunt infection (average incidence 3% to 20%) (Borgbjerg 1995; Drake 1998; Greenberg 2010; James 2014; Kestle 2011; Kestle 2016; Konstantelias 2015; Simon 2009); the highest proportion of infections is seen in infants (Bondurant 1995; Casey 1997). The symptoms associated with a shunt infection can be non‐specific, such as fever, nausea, lethargy (a lower level of consciousness), anorexia (a lack of appetite for food) or irritability. Symptoms of shunt infections in children tend to be more distinctive, such as high fever with or without concomitant meningitis, and rapid neurological deterioration. Up to 29% of individuals presenting with shunt malfunction have been shown to have a shunt infection, as confirmed by positive CSF cultures (a method to multiply micro‐organisms in order to determine the type of organism) (Greenberg 2010). Shunt infections can be treated by long‐term administration of antibiotics, but in most cases shunt revision is required (Greenberg 2010; Simon 2010). Both antibiotics and shunt revision can lead to longer hospital stays, additional complications and greater associated costs (Attenello 2010; Sciubba 2007); hence minimising shunt infections would be beneficial to both patients and to the healthcare system.
Description of the intervention
Currently, antibiotics used for the prevention of shunt infections can be administered in five ways: orally; intravenously; intrathecally; topically; and via the implantation of antibiotic‐impregnated shunt catheters. Antibiotics given via the oral route are used as add‐on therapy in the treatment of CSF‐shunt infections, but are rarely used to prevent CSF‐shunt infections (Frame 1984).
Intravenous pre‐operative antibiotics are widely used as shunt infection prophylaxis, and appear to lower the risk of such infections (Klimo 2014); however, these systemic antibiotics infiltrate the central nervous system poorly, and so intravenous antibiotics are often combined with antibiotics administered via one of the other routes. Ragel 2006 found that the addition of intrathecal gentamicin and vancomycin to intravenous cefazolin reduced the shunt infection rate to 0.42% (from 5.4% in the intrathecal gentamicin plus intravenous cefazolin administered to the control group).
Intrathecal antibiotics are usually administered intraoperatively; however, Moussa 2016 used a shunt containing a reservoir in which a prophylactic antibiotic was injected. They showed that an additional administration of antibiotics one week after surgery resulted in a lower shunt infection rate than intra‐operative administration alone. Another option is the administration of topical antibiotics. This route is partly similar to intrathecal administration but provides the opportunity of covering the entire drainage route (including the extracranial pathway), since the drain is drenched in an antibiotic agent.
A relatively new technique in the field of shunt infection prevention is the antibiotic‐impregnated catheter. These catheters, which are impregnated with two antibiotic agents, slowly release antibiotics over a period of days, and they have been shown to significantly reduce the rate of shunt infections (Konstantelias 2015). However, such catheters are relatively expensive when compared with the previously mentioned administration routes: for example the incremental cost of topical vancomycin are USD 10 per surgery versus USD 400 for antibiotic‐impregnated catheters (van Lindert 2018). In addition, Konstantelias 2015 found that antibiotic‐impregnated shunt catheters had a higher probability of colonisation by strains of bacteria that are more virulent than coagulase‐negative staphylococci (CoNS), which can result in a more severe infection. Another concern regarding the use of antibiotic‐impregnated shunt catheters was noted by James 2014, who found that individuals who needed shunt replacement after the implantation of an antibiotic‐impregnated shunt catheter were more prone to infections than those who were initially treated with other types of shunt.
How the intervention might work
Foreign materials, such as a shunt or an external drain, are prone to infection when placed in the body, and once infected, the management can be challenging. Antibiotic agents have a bactericide or bacteriostatic function that helps to eliminate bacterial infections. These functions are also useful when the prevention of bacterial colonisation is the aim. Although a ventriculoperitoneal shunt can be in situ for years, most shunt infections occur within two months of surgery (Greenberg 2010). The source of the infection is usually bacteria from the individual’s own skin (Yogev 1985). Hence contamination of the shunt takes place during, or early after, surgery, which suggests that the perioperative administration of antibiotic prophylaxis could be effective in the prevention of shunt infections.
Why it is important to do this review
CSF‐shunt infection is a major problem in individuals (including children) with hydrocephalus, with a reported proportion of shunt infection rates of 3% to 20% (Greenberg 2010). Shunt infections have a very high impact, both clinically (repeat surgery, prolonged hospitalisation, neurological deterioration) and economically (we estimated that each infection is associated with incremental costs equating to EUR 30,000). Hence a reduction in the shunt infection rate is urgently needed. Prophylactic antibiotics are currently the main prevention strategy in use; however, the best route of administration for the prevention of shunt infection remains to be determined (Ratilal 2008). This Cochrane Review has the potential to establish whether antibiotic prophylaxis has a positive effect on the proportion of CSF‐shunt infections and could direct which route of administration is the most effective. Some other aspects of antibiotic treatment (duration of treatment, dose and intervals between doses) are also unknown, but due to the potentially wide variety in these three factors, we did not include them in our review.
Objectives
To determine the effect of different routes of antibiotic prophylaxis (i.e. oral, intravenous, intrathecal, topical and via antibiotic‐impregnated catheters) on CSF‐shunt infections in persons treated for hydrocephalus using internalised CSF shunts.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised and quasi‐randomised controlled trials that studied the effect of antibiotic prophylaxis for the prevention of CSF‐shunt infection. We considered cluster randomised trials as eligible for inclusion but cross‐over trials were not taken into account since these are technically impossible to perform within the research question of this review. We excluded studies in which participants received more than one shunt simultaneously.
Types of participants
All individuals, of any age and gender, who underwent any type of internalised CSF‐shunt placement for the treatment of hydrocephalus. We imposed no restrictions with respect to the aetiology of hydrocephalus. We included studies that enrolled only a subset of relevant participants; we presented the data from these studies only for the relevant subset. If data on the subset of relevant participants could not be obtained, we excluded the study. We excluded individuals treated with external drains or temporary shunts.
Types of interventions
We included all types of antibiotics compared with standard care, placebo, or other active antibiotics. We included regimens as defined in primary studies irrespective of their dose, frequency and intensity and for any duration of therapy. We included studies investigating any of the following administration routes of antibiotic prophylaxis: oral; intravenous; topical; intrathecal; and via antibiotic‐impregnated shunt catheters.
Types of outcome measures
Primary outcomes
Number of participants experiencing shunt infection after administration of antibiotics.
We defined shunt infection as 'clinical and biochemical signs of infection in combination with a positive CSF culture'. We reported the proportion of participants who experienced shunt infection as counts and percentages.
All outcomes needed to occur within two years after shunt placement. We reported outcomes in three time frames: short term (< 30 days), medium term (30 days to 6 months), long term (> 6 months).
Secondary outcomes
No secondary outcomes were taken into account in this review.
Search methods for identification of studies
We conducted a systematic electronic search without restrictions on language, date or publication type, in line with the advice given in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefevbre 2011). If we identified studies published in a language other than English, we asked a professional translator to translate the text.
Electronic searches
We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL) (2017; Issue 12) in the Cochrane Library; (Appendix 1).
MEDLINE (PubMed) (1966 to 23 January 2018); (Appendix 2).
Embase (Embase.com) (1974 to 23 January 2018); (Appendix 3).
We searched for the terms in the title, abstract, keywords and controlled vocabularies.
Searching other resources
We then searched the following other resources.
The reference lists of all retrieved articles, texts and other reviews on the topic.
The ISRCTN registry (isrctn.com/), to identify any unpublished data.
Web of Science (webofscience.com), for forward citation search.
We also attempted to contact authors of included studies to obtain key missing data as needed.
Data collection and analysis
Selection of studies
Three review authors (SA, HB and EvL) independently screened titles and abstracts for inclusion with regard to our eligibility criteria, which were stored in a reference management software system. We obtained full‐text versions of the articles that met the eligibility criteria. The three review authors (SA, HB and EvL) again independently read these articles and listed those that did not meet the inclusion criteria in a 'Characteristics of excluded studies' table. We resolved disagreements by discussion or by referral to an independent researcher within our department when necessary.
Data extraction and management
Two review authors (SA and EvL) independently extracted the following data from included studies.
Date and country of study.
Study design.
Number of participants.
Demographic data.
Inclusion and exclusion criteria.
Antibiotic prophylaxis (type, administration route, frequency and doses of antibiotics).
Primary and secondary outcomes.
Methodological quality of the study.
We summarised all studies that met the inclusion criteria in a ’Characteristics of included studies’ table provided in Review Manager 5 (RevMan 5) and included details related to design, participants, interventions and outcomes (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors (SA and EvL) independently assessed the risk of bias of the included studies using the Cochrane 'Risk of bias' tool, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed the following domains of bias.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other biases.
After this independent risk of bias assessment, we resolved any disagreements by discussion with a third review author (HB).
We judged the overall risk of bias of each included study according to the following criteria.
Low risk of bias (plausible bias unlikely to seriously alter the results) if all the above items were met.
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more items were assessed as unclear.
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more items were not met.
Measures of treatment effect
We planned to perform meta‐analyses using the risk ratio as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Unit of analysis issues
We considered any of the following designs of included studies as having high potential for 'unit of analysis' issues.
Cluster‐randomisation.
Simultaneous multiple routes of antibiotic administration on each individual.
Repeated measurements (i.e. recurring infections in the same participant).
When appropriate controls were not used, we requested additional information from the authors and re‐analysed the data according to Chapters 9 (Deeks 2011) and 16 (Higgins 2011b) in the Cochrane Handbook for Systematic Reviews of Interventions. Hence for cluster‐randomised trials, we first checked whether an effective sample size could be calculated. If this was not possible, we excluded the study from the analysis. If a proper calculation of the effective sample size could be performed, we included the study in the meta‐analysis using the generic inverse‐variance method. We obtained the data for individual participants of studies that included repeated measurements. After obtaining such data, we carried out an analysis that included the whole follow‐up period for each participant (e.g. a time‐to‐event analysis).
Dealing with missing data
In the case of missing data we first contacted the authors and requested their database. In this way we hoped to receive missing data or determine whether the data are randomly or structurally missing. We then performed sensitivity analyses to assess how sensitive the results were to reasonable changes in the assumptions that were made. If the data were missing at random we analysed only the available data. If data were not missing at random we considered one of the following options.
Imputing the missing data with replacement values and treating these as if they were observed.
Imputing the missing data and accounting for the fact that these were imputed with uncertainty.
Using statistical models to allow for missing data and making assumptions about their relationship with the available data.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by critically appraising the included studies. When clinical and methodological heterogeneity was unlikely, we assessed statistical heterogeneity by performing a meta‐analysis. First, we assessed heterogeneity using the confidence interval of the forest plot; thereafter we took the Chi² test and I² statistic into account (Higgins 2011c).
We interpreted the I² statistic as follows.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: represents considerable heterogeneity.
When statistical heterogeneity was detected, we re‐assessed the individual studies in order to find the origin of the heterogeneity.
Assessment of reporting biases
We built a funnel plot in order to assess publication bias. We assessed selective outcome reporting bias by critically appraising the included studies.
Data synthesis
Since our research question was broad we used a random‐effects model in our meta‐analyses, though we used a fixed‐effect model in subgroup analysis when heterogeneity was low. If substantial statistical heterogeneity was present and the directon of effect was inconsistent across studies, we did not combine data in meta‐analysis but presented a narrative summary. We used RevMan 5 to perform analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses on:
administration route of antibiotics (i.e. oral; intravenous; intrathecal; topical; and via antibiotic‐impregnated catheters);
children and adults;
ventriculoperitoneal shunts and ventriculoatrial shunts;
individual type of antibiotic agent used.
When sufficient data were available we performed subgroup analyses according to the aetiology of hydrocephalus and gender. We conducted an indirect comparison analysis.
When heterogeneity was detected, we assessed the individual studies in order to find the origin of the heterogeneity. Furthermore, when moderate heterogeneity was detected we performed both a fixed‐effect and random‐effects model meta‐analysis.
Sensitivity analysis
If statistical heterogeneity was detected or if the eligibility of some studies in the meta‐analysis was dubious because they did not contain full details, we planned to conduct sensitivity analyses in order to check whether particular decisions or missing information that significantly influences the outcomes of this review can be identified, as described in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011c). For example: we analysed if the results of studies in which deficiencies were not likely to invalidate results, based on the study assessment in this review ('trials at low risk of overall bias'), were likely to show other results than studies that contained deficiencies that were more likely to invalidate results ('trials at uncertain and high risk of overall bias'). 'Trials at low risk of overall bias' were defined as studies in which risk of bias was considered low for at least five 'risk of bias' items. 'Trials at uncertain and high risk of bias' were defined as studies in which risk of bias was considered low for less than five 'risk of bias' items.
Overall quality of the body of evidence: 'Summary of findings' table
We reported the number of participants experiencing shunt infection after administration of antibiotics and judged the overall quality of the treatment effect according to the GRADE approach (GRADEpro GDT; Guyatt 2008). Two review authors (SA and EvL) rated the quality of evidence as 'high', 'moderate', 'low', or 'very low' using GRADEpro GDT. We resolved any disagreements by discussion or by referral to a third review author (HB) when necessary.
Results
Description of studies
Results of the search
A total of 1117 records were identified (CENTRAL: 19; MEDLINE: 411; Embase: 681; CINAHL: 1; ISRCTN registry (isrctn.com): 1; and clinicaltrials.gov: 4). After the removal of duplicates we screened titles and abstracts of 1106 records, which resulted in 21 records that met the eligibility criteria. Full text could not be obtained for three records, despite our emails to the corresponding authors and extensive library and database search. We excluded ten studies. In addition, we identified three articles through handsearch of reference lists. These three studies met eligibility criteria and were therefore included. Ultimately, 11 randomised controlled trials that were eligible according to the inclusion criteria provided data for the review (Figure 1).
1.
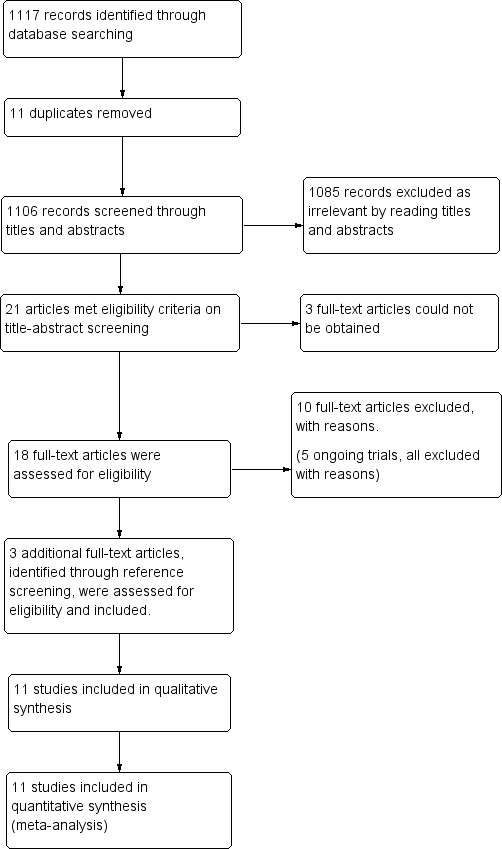
Study flow diagram.
In addition, we identified five ongoing trials through the clinical trials registries of which three were already completed (ISRCTN29451493; NCT00280904a; NCT00286104). All of them were excluded; two (ISRCTN29451493; NCT00286104) were excluded since only external drains were evaluated. NCT02600793 did not randomise and used external drains. NCT00280904a was not a randomised study and NCT01936272 did not include the use of antibiotic prophylaxis in their intervention. See Characteristics of excluded studies.
Included studies
We have presented the list and details of the included studies under Characteristics of included studies.
Participants
Of the 11 included trials, three included solely children (Blum 1989; Haines 1982; Rieder 1987). The other studies included participant of all ages.
Type of shunts
Most studies were limited to the evaluation of ventriculoperitoneal shunts. However five studies included participants with ventriculoatrial shunts (Blomstedt 1985; Blum 1989; Djindjian 1986; Lambert 1984; Schmidt 1985); of which one study contained four participants with a subduroperitoneal shunt (Blum 1989).
Intervention
The most commonly used administration route of antibiotic prophylaxis was intravenously (Blomstedt 1985; Blum 1989; Bullock 1988; Djindjian 1986; Haines 1982; Rieder 1987; Schmidt 1985; Wang 1984), followed by intrathecally (Bayston 1990; Lambert 1984). Administration via antibiotic impregnated catheters were used in one study (Govender 2003;). No studies regarding topical or oral administration of antibiotics were eligible for inclusion.
Controls
Generally, two varieties of control groups were used: standard care (Bayston 1990; Djindjian 1986; Govender 2003; Schmidt 1985); and placebo (Blomstedt 1985; Blum 1989; Bullock 1988; Haines 1982; Rieder 1987; Wang 1984). In the study of Blomstedt 1985 the placebo contained ethanol and propylene glycol. The Bayston 1990 trial did not define their standard of care because of high variability due to the multicentre design. One study compared povidone iodine to antibiotics (Lambert 1984).
Follow‐up
Follow‐up in included studies ranged from eight weeks to more than one year. The time at which shunt infections occurred was only reported in a few studies (Blomstedt 1985; Bullock 1988; Govender 2003; Schmidt 1985; Wang 1984).
Country
Three studies were performed in the UK (Bayston 1990; Govender 2003; Rieder 1987), two in Germany (Blum 1989; Lambert 1984). The other studies were performed in France (Djindjian 1986), the USA (Govender 2003; Haines 1982), Denmark (Schmidt 1985), Canada (Wang 1984), Finland (Blomstedt 1985), and South Africa (Bullock 1988; Govender 2003). Three studies were multicentre (Bayston 1990; Govender 2003; Schmidt 1985).
Defenition of infection
In all studies shunt infection was diagnosed based on clinical assessment, biochemistry and positive CSF culture.
Excluded studies
We have presented the list and descriptions of excluded studies under Characteristics of excluded studies. On the basis of full‐text assessment, we excluded ten publications, two of which were observational studies (Eymann 2008; Kumar 2016). We excluded an additional five studies as they did not report on shunt infections in internal shunts (Odio 1984; Tacconelli 2008; Walters 1992; Young 1987; Zentner 1995). Moreover, three studies were excluded since antibiotic prophylaxis were used in the control group (Moussa 2017; Nejat 2008; Theophilus 2011).
Risk of bias in included studies
All included studies were randomised controlled trials as described in the table of Characteristics of included studies. We have presented details of risk of bias of included studies in Figure 2 and Figure 3. Considering our predefined criteria (Assessment of risk of bias in included studies) we judged four out of 11 (36%) trials at unclear risk of bias (Blomstedt 1985; Bullock 1988; Rieder 1987; Wang 1984), while the remaining seven trials (64%) were scored at high risk of bias in one or more of the components assessed.
2.
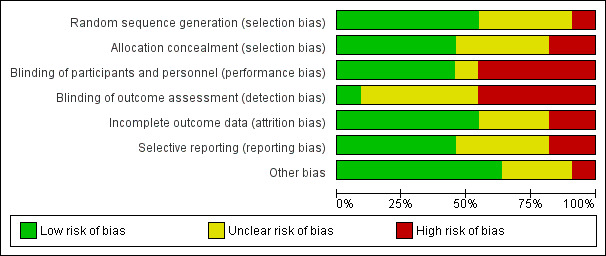
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
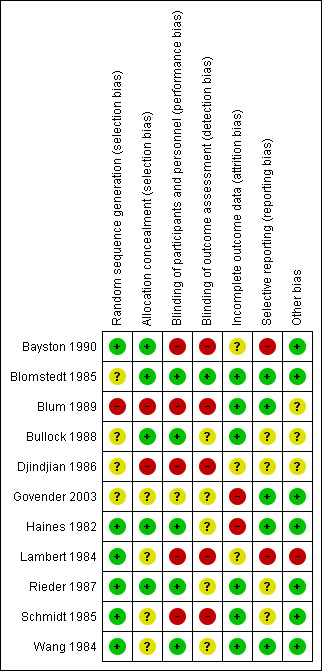
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered allocation concealment inappropriate in two studies (Blum 1989; Djindjian 1986). In the Blum 1989 trial they randomised their participants on date of birth; since a date of birth can be checked we considered the allocation concealment as inappropriate. Djindjian 1986 performed an open trial. We scored four studies as unclear risk of bias (Govender 2003; Lambert 1984; Schmidt 1985; Wang 1984).
Blinding
The risk of bias in blinding of participants and personnel remained unclear in one study (Govender 2003) and we considered it to be inadequately performed in five studies (Bayston 1990; Blum 1989; Djindjian 1986; Lambert 1984; Schmidt 1985). Blum 1989 performed a single blinded trial. In the Djindjian 1986 trial they performed an open trial. In the studies of Bayston 1990 and Schmidt 1985 no blinding on participants and personnel or outcome assessment was performed. We considered all five studies that had inadequate blinding on participants and personnel to be at high risk of bias for blinding their outcome assessment. One study scored low risk on blinding their outcome assessment (Blomstedt 1985), but most studies reported inadequately on their method of blinding their outcome assessment.
Incomplete outcome data
In five studies we had concerns about missing data. In three of these five studies the reason for exclusion was described (Blomstedt 1985; Bullock 1988; Wang 1984). Bullock 1988 presented a Chi² test to prove that missing participants were equally distributed between the comparison groups. Two studies reported that participants were lost to follow‐up (Govender 2003; Haines 1982). We were not able to track if these participants were allocated to the intervention group or the control group. Both studies reported that these participants did not suffer from infection at time of their latest follow‐up. We were not able to get the raw data and could therefore not determine whether data were missing at random.
Selective reporting
We considered selective reporting as a potential high risk of bias in two studies (Bayston 1990; Lambert 1984). They had no clear study protocol and no statistical analysis was performed. In four studies it was unclear whether reporting bias was likely to occur (Bullock 1988; Djindjian 1986; Rieder 1987; Schmidt 1985). We considered the remaining studies to have a low risk for reporting bias.
Other potential sources of bias
We considered one study to be at high risk for potential other sources of bias (Lambert 1984), since the dose of gentamicin was increased to 10 mg when ventricles were dilated while no cut‐off point for dilatation was described. We could find no documentation about the number of participants with dilatated ventricles. We scored three studies as unclear (Blum 1989; Bullock 1988; Djindjian 1986). A funnel plot was established in order to check for publication bias (Figure 4). As expected, precise studies were located near the average, but all less precise studies showed a positive effect of antibiotic prophylaxis on the proportion of participants that experienced CSF‐shunt infection. It is unlikely that the asymmetry in this funnel plot is due to publication bias, but rather the result of systematic difference between studies of higher and lower precision, also known as small‐study effects.
4.
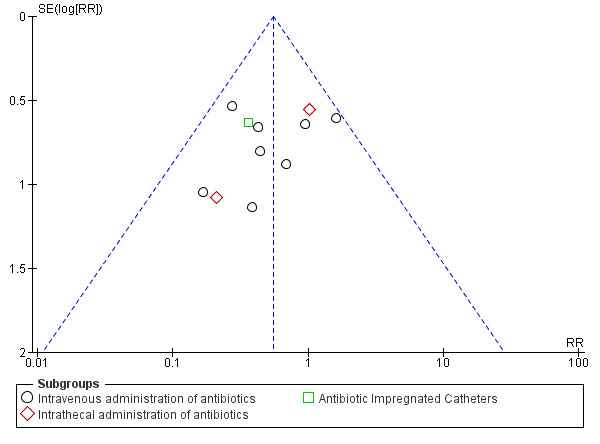
Funnel plot of comparison: 1 Treatment benefit of antibiotic prophylaxis versus no antibiotic prophylaxis, outcome: 1.1 Number of participants experiencing shunt infection after administration of antibiotics.
Effects of interventions
See: Table 1
Table 1 provides a summary of the risk estimates for shunt infection and the grading of the evidence.
We performed four separate meta‐analyses in order to compare the effect of antibiotic prophylaxis on the number of participants who had shunt infections. We compared different administration routes (11 RCTs, 1109 participants), children and adults (6 RCTs, 561 participants), ventriculoperitoneal and ventriculoatrial shunt systems (8 RCTs, 905 participants) and different antibiotic agents (11 RCTs, 1109 participants).
We assessed heterogeneity between trials in three steps: forest plot assessment; using the I² statistic; and using the Chi² test. Based on the combination of these three parameters we used a fixed‐effect model in Analysis 1.1 and Analysis 2.1 and a random‐effects model in Analysis 3.1 and Analysis 4.1.
1.1. Analysis.
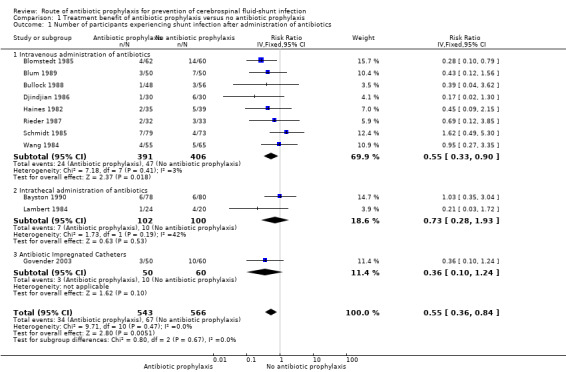
Comparison 1 Treatment benefit of antibiotic prophylaxis versus no antibiotic prophylaxis, Outcome 1 Number of participants experiencing shunt infection after administration of antibiotics.
2.1. Analysis.
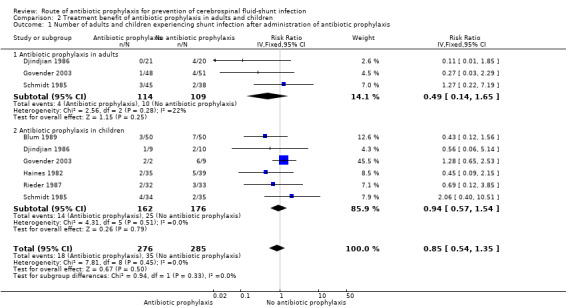
Comparison 2 Treatment benefit of antibiotic prophylaxis in adults and children, Outcome 1 Number of adults and children experiencing shunt infection after administration of antibiotic prophylaxis.
3.1. Analysis.
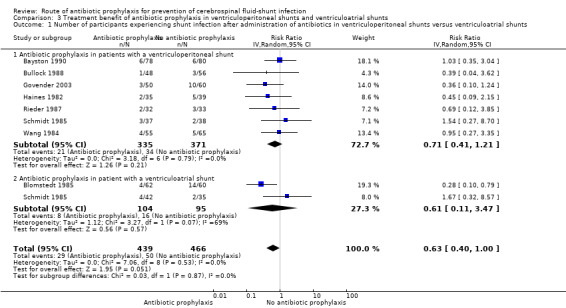
Comparison 3 Treatment benefit of antibiotic prophylaxis in ventriculoperitoneal shunts and ventriculoatrial shunts, Outcome 1 Number of participants experiencing shunt infection after administration of antibiotics in ventriculoperitoneal shunts versus ventriculoatrial shunts.
4.1. Analysis.
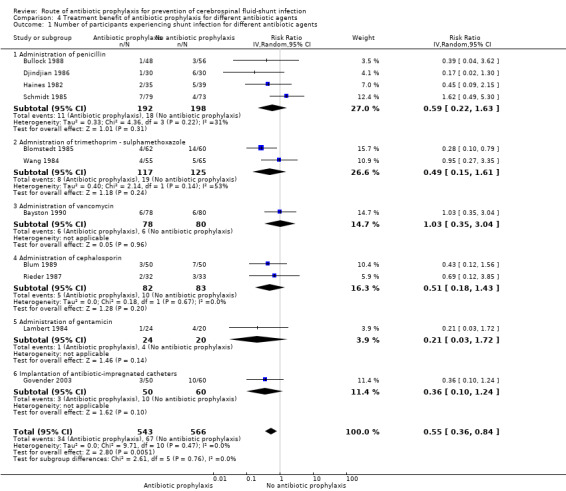
Comparison 4 Treatment benefit of antibiotic prophylaxis for different antibiotic agents, Outcome 1 Number of participants experiencing shunt infection for different antibiotic agents.
Number of participants experiencing CSF‐shunt infection
Eleven studies with 1109 participants were available. This analysis showed a positive effect of antibiotic prophylaxis on the number of shunt infections (RR 0.55, 95% CI 0.36 to 0.84), meaning a 45% reduction in the number of participants who had shunt infection compared with standard care or placebo. The heterogeneity I² for this meta‐analysis was 0%, which is low. Using GRADE criteria, we considered the evidence of very low certainty, downgrading two levels because we judged it at serious risk of suffering from selection bias, performance bias, and detection bias. We downgraded an additional level for indirectness.
Subgroup analysis: route of antibiotic prophylaxis (Analysis 1.1)
Intravenous
After analysing the eight included trials (797 participants) that used intravenous antibiotic prophylaxis, we found an overall statistical significance effect on the risk of shunt infections (RR 0.55, 95% CI 0.33 to 0.90). Looking at the individual trials, one trial was significant and favoured the intervention group (Blomstedt 1985). One trial favoured the control group (Schmidt 1985); and the other trials favoured the intervention group, though without significance. The heterogeneity I² for this meta‐analysis was 3%. This result indicates that the relative risk of shunt infection is reduced by 45%.
Intrathecal
We analysed two studies that used intrathecal antibiotics as intervention in this subgroup (Bayston 1990; Lambert 1984). We found no significant effect of intrathecal antibiotic prophylaxis on the risk of shunt infection (RR 0.73, 95% CI 0.28 to 1.93). One of the infections in the trial of Bayston 1990 occurred after trans‐labyrinthine removal of an acoustic neuroma one month after shunt placement. It is not described in which comparison group this participant was included. Heterogeneity between these studies was moderate (I² = 42%). Both studies included ventriculoperitoneal shunts without age limitations, but in Lambert 1984 both ventriculoperitoneal and ventriculoatrial shunts were included. Lambert 1984 and Bayston 1990 were considered at high risk of bias. These uncertainties and varying factors contributed to the moderate level of heterogeneity between these studies.
Topical
No studies regarding the use of topical antibiotic prophylaxis in the prevention of shunt infection met the eligibility criteria.
Oral
No studies regarding the use of oral antibiotic prophylaxis in the prevention of shunt infection met the eligibility criteria.
Antibiotic‐impregnated catheters
One study used antibiotic‐impregnated catheters (Govender 2003). This study did not demonstrate a significant effect on the number of participants that experienced CSF‐shunt infection (RR 0.36, 95% CI 0.10 to 1.24)
Subgroup analysis: adults and children (Analysis 2.1)
Since children seem to be more prone to infection, we analysed differences in the effect of antibiotic prophylaxis between children and adults. Most included trials enrolled participants of any age and therefore we checked if we could find any differentiation on age within the trial results. If this was the case we extracted data on both subgroups and recalculated the proportion of participants who had shunt infections when necessary. We included three studies that reported on the number of children that experienced shunt infection (Djindjian 1986; Govender 2003; Schmidt 1985) . I² was 22%. In the trial of Djindjian 1986 participants were divided into age groups (< 6 years old; 6 to 60 years old; and over 60 years old). It was unclear how many children were included in the '6 to 60' age group; however since the only two infections occurred in adults we added this group to the adult section in the analysis. Six studies reported on the number of adults that experienced shunt infection (338 participants). The test for subgroup differences showed no evidence of a difference (p = 0.33) between children (RR 0.94, 95% CI 0.57 to 1.54) and adults (RR 0.49, 95% CI 0.14 to 1.65).
Subgroup analysis: ventriculoperitoneal shunt and ventriculoatrial shunt (Analysis 3.1)
We included 7 studies (706 participants) in the ventriculoperitoneal shunt group. There was no heterogeneity in this meta‐analysis (I² = 0%). The ventriculoatrial shunt group consisted of two studies (Blomstedt 1985; Schmidt 1985). Heterogeneity in these two studies was substantial (I² = 69%). Schmidt 1985 was not blinded and included both ventriculoatrial shunts and external ventriculostomies. In Blomstedt 1985 only participants older than 12 years were included. The test for subgroup differences showed no evidence of a difference (p = 0.87) between the ventriculoperitoneal shunt group (RR 0.71, 95% CI 0.41 to 1.21) and the ventriculoatrial shunt group (RR 0.61, 95% CI 0.11 to 3.47).
Subgroup analysis: kind of antibiotic used (Analysis 4.1)
Penicillin
We included four studies in this analysis (Bullock 1988; Djindjian 1986; Haines 1982; Schmidt 1985). We found no combined statistically significant effect (RR 0.59, 95% CI 0.22 to 1.63).
Trimethoprim‐sulphamethoxazole
We evaluated two studies in this subgroup (Blomstedt 1985; Wang 1984), of which one showed a statistically significant result (Blomstedt 1985). However, the overall 95% confidence interval was not significant (RR 0.49, 95% CI 0.15 to 1.61).
Vancomycin
We analysed one study that used vancomycin as antibiotic prophylaxis in this subgroup (Bayston 1990), this study did not show a significant effect (RR 1.03, 95% CI 0.35 to 3.04). The risk of bias in this study was high.
Cephalosporin
We analysed two studies in this subgroup (Blum 1989; Rieder 1987). Neither of these showed a statistically significant effect and no overall statistical effect could be demonstrated (RR 0.51, 95% CI 0.18 to 1.43).
Gentamicin
Lambert 1984 used gentamicin as antibiotic agent. Results of this study were not statistically significant (RR 0.21, 95% CI 0.03 to 1.72).
Antibiotic impregnated catheters
Govender 2003 used antibiotic‐impregnated catheters (RR 0.36, 95% CI 0.10 to 1.24).
Other results
Only three studies reported — briefly — on the aetiology of hydrocephalus (Blomstedt 1985; Bullock 1988; Rieder 1987). The other 8 studies reported either nothing (Djindjian 1986; Haines 1982; Lambert 1984; Wang 1984), or that all aetiologies were eligible for inclusion (Bayston 1990; Blum 1989; Govender 2003; Schmidt 1985;). Data on gender were not presented in four studies (Bayston 1990; Blomstedt 1985; Djindjian 1986; Lambert 1984). Although the other studies did report on gender, only two studies reported the proportion of participants that experienced CSF‐shunt infection related to gender (Rieder 1987; Schmidt 1985). Blum 1989 reported that no significant correlation was found between shunt infection and gender.
The onset of shunt infection was mentioned in five studies (Blomstedt 1985; Bullock 1988; Govender 2003; Schmidt 1985; Wang 1984). In these studies it was seen that participants who did not receive antibiotic prophylaxis were more likely to develop shunt infections within 30 days after surgery. In the antibiotic prophylaxis group infections were more distributed between the different time frames. Exact numbers or means are not displayed here since a wide variety of presentation forms were used among the articles.
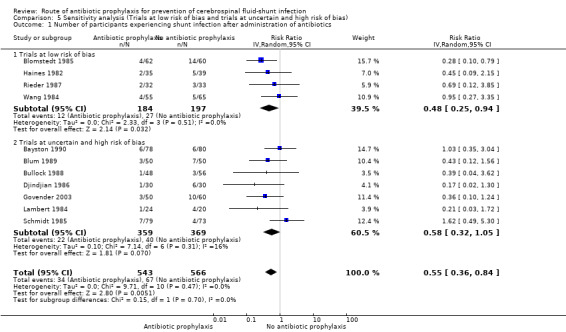
Sensitivity analysis
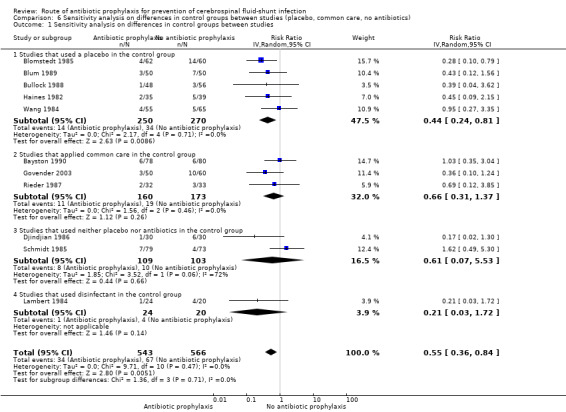
The first sensitivity analysis that was performed contained all studies divided into low risk of bias and uncertain or high risk of bias. In the low risk of bias group four studies used intravenous administration and one used topical administration. A significant effect in favour of antibiotic prophylaxis was found (RR 0.48, 95% CI 0.25 to 0.94). Blomstedt 1985 had considerable impact on the overall significance level in the low risk of bias group, since no significant effect was found after excluding this trial from the analysis (RR 0.70, 95% CI 0.30 to 1.65). Studies in the uncertain and high risk of bias group were just without range of a statistically significant result (RR 0.58, 95% CI 0.32 to 1.05). A second sensitivity analysis was performed regarding the difference in control groups. It showed that only studies that used placebo as control had a significant effect of antibiotics prophylaxis on shunt infection (RR 0.44, 95% CI 0.24 to 0.81).
Point estimate of the control group
We performed a point estimate of the control group to check whether the incidence of shunt infections has declined over time without intervention. Possible causes for this decline could be the improvement of hygiene protocols or improved instrumentation. This decline over time would cause an underestimation of the effect of studies that are performed more recently compared to older studies. However, no decline of infections in the control group over time was found (data not shown).
Discussion
Summary of main results
It is uncertain whether antibiotic prophylaxis had a positive effect on reducing the number of participants who experienced CSF‐shunt infection, since the results of this review are equivocal and based on low quality evidence. Most of the included studies compared intravenous administration of antibiotics versus placebo or standard care; two studies used intrathecal antibiotics in the intervention group and one antibiotic‐impregnated catheters. Only intravenous administration of antibiotic prophylaxis showed a significant effect on the number of participants who had shunt infections. However, this was the only subgroup that contained more than two studies. The antibiotic‐impregnated catheter group contained one study that showed no significant effect on the number of shunt infections.
Looking at the difference in effect between adults and children, no evidence of a significant effect in studies regarding adults and those regarding children was found. Although the result in adults was not statistically significant, probably due to the low number of included participants in the analysis, a risk reduction of 51% might be clinically important. Reporting on timing of onset of infection was low. However, participants who did not receive antibiotic prophylaxis were more likely to develop shunt infections within 30 days after surgery which suggest that antibiotic prophylaxis might have an effect on shunt infection that occur within a short period after surgery. Since data on the onset of infection in this review is very low, more research needs to be performed in order to adequately analyse the effect of antibiotic prophylaxis on the timing of onset of infection.
Due to the limited number of RCTs or limited description of outcomes, not every subgroup analysis contained a sufficient number of studies to draw grounded conclusions. In the evaluation of different administration routes only one study on antibiotic‐impregnated catheters (Govender 2003) was available. In the meta‐analysis on ventriculoperitoneal and ventriculoatrial shunts only two studies were eligible for evaluation of ventriculoatrial shunts (Blomstedt 1985; Schmidt 1985), since other studies did not report on shunt infections in ventriculoatrial shunts separately. In the final meta‐analysis regarding the kind of antibiotic agent, only one subgroup — penicillin — comprised more than two studies. Due to the variety of antibiotic agents in combination with the low number of included studies per antibiotic agent, no conclusions could be drawn on which is the most effective in preventing shunt infections.
Overall completeness and applicability of evidence
This review shows a significant effect for the use of antibiotic prophylaxis for the prevention of CSF‐shunt infections. However, the methods in the included studies were very heterogenic on, for example, the age of participants and aetiology of hydrocephalus, the kind and dosage of antibiotic agents, and the time of follow‐up. Moreover, most study methods were marginally described, especially in the older studies. Due to the limited description of methods and results, some parameters could not be studied. Although the overall effect was statistically significant, the only subgroup that showed a statistically significant effect was intravenous administration. A lot of clinically important aspects remain unclear, i.e. in which age group antibiotic prophylaxis are recommended, what kind of antibiotic agent is most effective and for what kind of drains antibiotic prophylaxis might work.
Overall, antibiotic prophylaxis are effective in preventing CSF‐shunt infection. It remains unclear what administration route or agent is best for preventing shunt infections. Heterogeneity between studies limits the external validity and therefore no generalised conclusion can be drawn.
In this review, we performed a thorough search in various databases. However, most included studies were published before the year 2000 and they may not reflect the change in practice over time.
Quality of the evidence
The quality of evidence for the overall effect of the administration of antibiotic prophylaxis, presented in the 'Summary of findings' table, was low. We downgraded the level of evidence two levels. Downgrading of one level was due to indirectness of evidence, since control groups differed a lot. For example, Bayston 1990 compared the used of antibiotic prophylaxis to current practice in a multicentre design, it was not formulated whether one of the centre's standard care enclosed the use of antibiotics. We downgraded another two levels because we considered studies at serious or unclear risk of bias. The quality of evidence for the administration of intravenous antibiotic prophylaxis was downgraded two levels, since the control groups of included studies differed a lot and again the risk of bias was considerably. Also in the studies that administered antibiotic prophylaxis intrathecally, the risk of bias was considered at serious risk, therefore the quality of evidence was downgraded by two levels. One study implanted antibiotic impregnated catheters (Govender 2003). This study was was considered at serious or unclear risk of bias at five out of seven items, therefore the level of evidence was downgraded two levels. Besides the risk of bias, the level of evidence was downgraded one more level, since the subgroup consisted of only one study and this study consisted of only 110 inclusions.
Potential biases in the review process
This review was based on a systematic review of 11 small trials of antibiotic prophylaxis for prevention of shunt infection. There was a statistically significant reduction in shunt infection in participants who received antibiotic prophylaxis. Although we performed a comprehensive search without any restrictions in a variety of databases and performed a comprehensive search for additional records in the reference lists of included studies in order to minimize publication bias, some data could have been missed. Besides, no full text could be obtained in three records. All stages and decisions were performed by at least two of three authors in order to minimize bias in the review process. When disagreement occurred it was discussed in detail within the team. Since we were not able to get raw data, some results may be biased due to incomplete outcome data.
Moreover, randomised controlled trials are rare in the field of CSF‐shunt surgery. Most of the studies performed on CSF‐shunt infection are cohort studies that either focus on incidence or risk factors. This means that valuable data could be missed in a systematic review focusing on RCTs.
Agreements and disagreements with other studies or reviews
Ratilal 2008 reviewed the use of intravenous antibiotic prophylaxis and antibiotic‐impregnated catheters in both internal and external ventricular drainage. They found a significant effect of intravenous antibiotic prophylaxis for internal ventricular shunts but no significant effect was found for external ventricular drains. Besides, no conclusions can be drawn on the use of antibiotic‐impregnated internal shunts, since only one randomised controlled trial was included. Although the objective of the Ratilal 2008 review differs, the sub‐conclusion that the use of antibiotic prophylaxis has a positive effect on lowering CSF‐shunt infection is shared.
Authors' conclusions
Implications for practice.
Antibiotic prophylaxis may have a positive effect on lowering the number of participants who had shunt infections. However, the quality of evidence, assessed using GRADE, was very low. The low level of evidence within this review is the result of few eligible studies and the studies that were eligible were at high or unclear risk of bias. Moreover, only intravenous administration showed a statistically significant effect, possible because this was the only subgroup that consisted of more than two studies. In other words, other routes of administration might also prevent shunt infection but the amount of data in this review was insufficient to show this possible effect. Moreover, no unambiguous comparison between studies could be made due to methodological differences. This makes it hard to establish a baseline effect of antibiotic prophylaxis regimes on the prevention of shunt infections.
Implications for research.
Neurosurgery is a small clinical field and therefore it will be hard to include the number of patients needed to perform a high‐quality clinical trial. Besides this limitation, half of shunt surgeries are performed in an emergency setting, which makes obtaining proper informed consent difficult. The results of the review should be seen as hypothesis‐generating rather than definitive and the results should be confirmed in adequately powered trials or large multicenter studies in order to obtain high‐quality evidence in the field of ventricular shunt infection prevention. Uniformity in the antibiotic prophylaxis regime and control group are the keystone for a potential high‐quality study.
Acknowledgements
We are grateful to Dr Elizabeth Royle, Cochrane Central Executive Team, for reviewing the review.
Appendices
Appendix 1. CENTRAL
#1hydrocephal*:ti,ab,kw #2MeSH descriptor: [Hydrocephalus] explode all trees #3aqu?ductal stenos?s:ti,ab,kw #4#1 or #2 or #3 #5MeSH descriptor: [Cerebrospinal Fluid Shunts] explode all trees #6(shunt* or catheter*):ti,ab,kw #7#5 or #6 #8#4 and #7 #9MeSH descriptor: [Anti‐Bacterial Agents] explode all trees #10antibiotic* or anti‐bacterial or antibacterial or anti near/4 bacterial:ti,ab,kw #11MeSH descriptor: [Antibiotic Prophylaxis] explode all trees #12MeSH descriptor: [Vancomycin] explode all trees #13vancomycin:ti,ab,kw #14vancomicin:ti,ab,kw #15MeSH descriptor: [Rifampin] explode all trees #16rifampicin:ti,ab,kw #17rifampin:ti,ab,kw #18MeSH descriptor: [Gentamicins] explode all trees #19gentam?cin:ti,ab,kw #20MeSH descriptor: [Methicillin] explode all trees #21methicillin or meticillin or methycillin or metycillin:ti,ab,kw #22MeSH descriptor: [Cefazolin] explode all trees #23cephazolin*:ti,ab,kw #24cefazolin*:ti,ab,kw #25#9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 #26[mh staphylococcus] or [mh streptococcus] #27s. aureus:ti,ab,kw #28st. aureus:ti,ab,kw #29staphylococcus aureus:ti,ab,kw #30s. epidermidis:ti,ab,kw #31st. epidermidis:ti,ab,kw #32staphylococcus epidermidis:ti,ab,kw #33bacterial infection*:ti,ab,kw #34bacterem*:ti,ab,kw #35(gram‐negative bacterial infection*):ti,ab,kw #36(gram negative bacterial infection*):ti,ab,kw #37(gram‐positive bacterial infection*):ti,ab,kw #38(gram positive bacterial infection*):ti,ab,kw #39staphylococ* infection*:ti,ab,kw #40streptococ* infection*:ti,ab,kw #41(catheter‐related infection* or catheter‐associated infection* or catheter* infection or prosthes*‐related infection* or prosthes* infection*):ti,ab,kw #42(shunt‐related infection* or shunt‐associated infection* or shunt* infection*):ti,ab,kw #43MeSH descriptor: [Infection] this term only #44MeSH descriptor: [Catheter‐Related Infections] this term only #45MeSH descriptor: [Prosthesis‐Related Infections] explode all trees #46MeSH descriptor: [Sepsis] explode all trees #47sepsis or blood poisoning or shock or toxemia* or circulatory failure or pyohemia* or pyemia or pyaemia or septicemia or circulatory collapse:ti,ab,kw #48MeSH descriptor: [Bacterial Infections] explode all trees #49#26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 #50#8 and #25 and #49
Appendix 2. MEDLINE
1 hydrocephal*.ti,ab,kf. (23714) 2 hydrocephalus/ or dandy‐walker syndrome/ or hydrocephalus, normal pressure/ (21817) 3 aqu?ductal stenos?s.ti,ab,kf. (674) 4 cerebrospinal fluid shunts/ or ventriculoperitoneal shunt/ (9913) 5 (shunt* or catheter*).ti,ab,kf. (235894) 6 exp Anti‐Bacterial Agents/ (636809) 7 exp Vancomycin/ (12005) 8 van?om?cin.ti,ab,kf,rn. (25325) 9 exp Rifampin/ (16257) 10 rifamp??in.ti,ab,kf,rn. (26494) 11 exp Gentamicins/ (18172) 12 gentam#cin.ti,ab,kf,rn. (24315) 13 exp Methicillin/ (3675) 14 met??cillin*.ti,ab,kf,rn. (29932) 15 exp Cefazolin/ (2584) 16 ce??azolin*.ti,ab,kf,rn. (4945) 17 (antibiotic* or anti‐bacterial or antibacterial or (anti adj4 bacterial)).ti,ab,kf. (333911) 18 exp Antibiotic Prophylaxis/ (11911) 19 exp Staphylococcus/ or exp streptococcus/ (157073) 20 (staphylococcus epidermidis or staphylococcus aureus).ti,ab,kf. (90371) 21 (s? aureus or s? epidermidis).ti,ab,kf. (34110) 22 (bacterial adj5 infection*).ti,ab,kf. (46788) 23 bacterem*.ti,ab,kf. (22597) 24 (gram‐negative adj4 bacterial adj4 infection*).ti,ab,kf. (1021) 25 (gram‐positive adj4 bacterial adj4 infection*).ti,ab,kf. (465) 26 staphylococ* infection*.ti,ab,kf. (6916) 27 ((shunt* or prosthes* or catheter*) adj4 infection*).ti,ab,kf. (10617) 28 pyohemia*.ti,ab,kf. (8) 29 py?emia*.ti,ab,kf. (161) 30 blood poisoning*.ti,ab,kf. (25) 31 (circulatory adj3 (collaps or failure)).ti,ab,kf. (2252) 32 shock.ti,ab,kf. (159681) 33 sepsis.ti,ab,kf. (80390) 34 toxemia*.ti,ab,kf. (5853) 35 septic?emia*.ti,ab,kf. (19623) 36 bacterial infections/ or meningitis, bacterial/ or gram‐negative bacterial infections/ or gram‐positive bacterial infections/ or staphylococcal infections/ or streptococcal infection/ or infection/ or catheter‐related infections/ or prosthesis‐related infections/ or sepsis/ or bacteremia/ or endotoxemia/ or shock, septic/ (284371) 37 1 or 2 or 3 (31047) 38 4 or 5 (238246) 39 37 and 38 (10565) 40 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (802056) 41 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 (636632) 42 39 and 40 and 41 (397)
Appendix 3. Embase
1 hydrocephal*.ti,ab,kw. (30549) 2 aqu?ductal stenos?s.ti,ab,kw. (889) 3 brain ventricle peritoneum shunt/ or cerebrospinal fluid shunting/ (12536) 4 (shunt* or catheter*).ti,ab,kw. (327207) 5 hydrocephalus/ or brain aqueduct stenosis/ or brain ventricle dilatation/ or communicating hydrocephalus/ or congenital hydrocephalus/ or costello syndrome/ or dandy walker syndrome/ or normotensive hydrocephalus/ or obstructive hydrocephalus/ or walker warburg syndrome/ (43875) 6 exp antibiotic agent/ (1189546) 7 (antibiotic* or anti‐bacterial or antibacterial or (anti adj4 bacterial)).ti,ab,kw. (425175) 8 exp vancomycin/ or vancomycin derivative/ (75963) 9 van?om?cin.ti,ab,kw,rn. (80189) 10 exp rifampicin/ (80059) 11 rifamp??in.ti,ab,kw,rn. (83132) 12 exp gentamicin/ (95859) 13 gentam#cin.ti,ab,kw,rn. (99462) 14 exp meticillin/ (22727) 15 met??cillin*.ti,ab,kw,rn. (45463) 16 exp cefazolin/ (23856) 17 ce??azolin*.ti,ab,kw,rn. (24421) 18 exp antibiotic prophylaxis/ (26990) 19 exp staphylococcus/ or exp streptococcus/ (279482) 20 (staphylococcus aureus or staphylococcus epidermidis).ti,ab,kw. (110835) 21 s? aureus.ti,ab,kw. (40211) 22 s? epidermidis.ti,ab,kw. (5419) 23 (bacterial adj5 infection*).ti,ab,kw. (62753) 24 bacterem*.ti,ab,kw. (28575) 25 ((gram‐negative adj4 bacterial adj4 infection*) or (gram‐positive adj4 bacterial adj4 infection*)).ti,ab,kw. (1765) 26 staphylococ* infection*.ti,ab,kw. (4619) 27 ((catheter* or shunt* or prosthes*) adj4 infection*).ti,ab,kw. (15124) 28 sepsis.ti,ab,kw. (120941) 29 pyohemia*.ti,ab,kw. (4) 30 py?emia*.ti,ab,kw. (115) 31 septic?emia.ti,ab,kw. (21677) 32 blood poisoning*.ti,ab,kw. (25) 33 (circulatory adj3 (collaps or failure)).ti,ab,kw. (3161) 34 shock.ti,ab,kw. (199561) 35 toxemia*.ti,ab,kw. (3996) 36 bacterial infection/ or infection/ or bacterial meningitis/ or gram negative infection/ or gram positive infection/ or staphylococcus infection/ or streptococcus infection/ or endotoxemia/ or exp device infection/ (742628) 37 bacteremia/ or sepsis/ or septic shock/ or septicemia/ (216240) 38 1 or 2 or 5 (48510) 39 3 or 4 (331237) 40 38 and 39 (14764) 41 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (1371513) 42 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 (1302104) 43 40 and 41 and 42 (909)
Data and analyses
Comparison 1. Treatment benefit of antibiotic prophylaxis versus no antibiotic prophylaxis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants experiencing shunt infection after administration of antibiotics | 11 | 1109 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.36, 0.84] |
| 1.1 Intravenous administration of antibiotics | 8 | 797 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.33, 0.90] |
| 1.2 Intrathecal administration of antibiotics | 2 | 202 | Risk Ratio (IV, Fixed, 95% CI) | 0.73 [0.28, 1.93] |
| 1.3 Antibiotic Impregnated Catheters | 1 | 110 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.10, 1.24] |
Comparison 2. Treatment benefit of antibiotic prophylaxis in adults and children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of adults and children experiencing shunt infection after administration of antibiotic prophylaxis | 6 | 561 | Risk Ratio (IV, Fixed, 95% CI) | 0.85 [0.54, 1.35] |
| 1.1 Antibiotic prophylaxis in adults | 3 | 223 | Risk Ratio (IV, Fixed, 95% CI) | 0.49 [0.14, 1.65] |
| 1.2 Antibiotic prophylaxis in children | 6 | 338 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.57, 1.54] |
Comparison 3. Treatment benefit of antibiotic prophylaxis in ventriculoperitoneal shunts and ventriculoatrial shunts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants experiencing shunt infection after administration of antibiotics in ventriculoperitoneal shunts versus ventriculoatrial shunts | 8 | 905 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.40, 1.00] |
| 1.1 Antibiotic prophylaxis in patients with a ventriculoperitoneal shunt | 7 | 706 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.41, 1.21] |
| 1.2 Antibiotic prophylaxis in patient with a ventriculoatrial shunt | 2 | 199 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.11, 3.47] |
Comparison 4. Treatment benefit of antibiotic prophylaxis for different antibiotic agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants experiencing shunt infection for different antibiotic agents | 11 | 1109 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.36, 0.84] |
| 1.1 Administration of penicillin | 4 | 390 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.22, 1.63] |
| 1.2 Admnistration of trimethoprim ‐ sulphamethoxazole | 2 | 242 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.15, 1.61] |
| 1.3 Administration of vancomycin | 1 | 158 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.35, 3.04] |
| 1.4 Administration of cephalosporin | 2 | 165 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.18, 1.43] |
| 1.5 Administration of gentamicin | 1 | 44 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.03, 1.72] |
| 1.6 Implantation of antibiotic‐impregnated catheters | 1 | 110 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.10, 1.24] |
Comparison 5. Sensitivity analysis (Trials at low risk of bias and trials at uncertain and high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants experiencing shunt infection after administration of antibiotics | 11 | 1109 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.36, 0.84] |
| 1.1 Trials at low risk of bias | 4 | 381 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.25, 0.94] |
| 1.2 Trials at uncertain and high risk of bias | 7 | 728 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.32, 1.05] |
5.1. Analysis.
Comparison 5 Sensitivity analysis (Trials at low risk of bias and trials at uncertain and high risk of bias), Outcome 1 Number of participants experiencing shunt infection after administration of antibiotics.
Comparison 6. Sensitivity analysis on differences in control groups between studies (placebo, common care, no antibiotics).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis on differences in control groups between studies | 11 | 1109 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.36, 0.84] |
| 1.1 Studies that used a placebo in the control group | 5 | 520 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.24, 0.81] |
| 1.2 Studies that applied common care in the control group | 3 | 333 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.31, 1.37] |
| 1.3 Studies that used neither placebo nor antibiotics in the control group | 2 | 212 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.07, 5.53] |
| 1.4 Studies that used disinfectant in the control group | 1 | 44 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.03, 1.72] |
6.1. Analysis.
Comparison 6 Sensitivity analysis on differences in control groups between studies (placebo, common care, no antibiotics), Outcome 1 Sensitivity analysis on differences in control groups between studies.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bayston 1990.
| Methods | Randomized, controlled multicentre study Method of randomisation: computer‐generated random numbers Kind of shunt: ventriculoperitoneal shunts Location: United Kingdom Follow‐up: minimally 1 year Duration: 2.5 years |
|
| Participants | Inclusion: adults and children undergoing insertion or revision of ventriculoperitoneal shunts Exclusion: therapeutic antibiotic use, reconsidering during operation that the patient did not need a ventriculoperitoneal shunt Treatment group: 78; control: 80 |
|
| Interventions | Vancomycin administered into the ventricular system during surgery at a standard dose of 10 mg Controls received the standard care for the centre they were admitted to |
|
| Outcomes | Proportion of participants who had ventriculoperitoneal shunt infection, no timing of outcome measurement described | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was achieved by the use of centrally issued computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | "printed on a card and obscured in such a way that the next numbers could not be seen" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No apparent missing outcome data described |
| Selective reporting (reporting bias) | High risk | Due to study abortion the number of participants included was 158 instead of 712; this makes the study highly underpowered and therefore at risk for reporting bias. Authors waive statistical analysis due to the low number of inclusions |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Blomstedt 1985.
| Methods | Randomized, controlled Method of randomisation: unclear Kind of shunt: ventriculoatrial shunts Location: Finland Follow‐up: for a minimum of 6 months Duration: 38 months |
|
| Participants | Inclusion: all patients undergoing ventriculoatriostomy or an external ventriculostomy were included in the trial Exclusion: patients under 12 years of age, patients allergic to sulfamethoxazole or trimethoprim, patients who received antibiotics during the preceding week and patients who already had a shunt in place when the trial started Treatment: 62, control: 60 |
|
| Interventions | Trimethoprim (80 mg) and sulphamethoxazole (400 mg) versus plain vehicle (sodium hydroxide, dietholamin, sodium pyrosulfis, ethanol, phenylcarbinol and propylengeglycol mixed in sterile water) Intravenous administration was performed just before surgery and 3 times postoperatively with intervals of 12 hours |
|
| Outcomes | Proportion of participants who had shunt infection Time between surgery and infection was up to 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Ampoules filled with trimethoprim‐sulphamethoxazole or placebo were coded by Hoffmann‐La Roche (supplier) and ampoules were administered to every participant just before surgery |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Clinicians and pharmacist did not know which group a particular number belonged" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | After all the data had been collected and fed into a computer file the key to the code was given to the investigators by Hoffmann‐La Roche |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26 exclusions, well defined in text |
| Selective reporting (reporting bias) | Low risk | No study protocol available but it is clear that the published report includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Blum 1989.
| Methods | Randomized, controlled Method of randomisation: date of birth Kind of shunt: ventriculoperitoneal shunts, ventriculoatrial shunts and subduro‐peritoneal shunts Location: Germany Follow‐up: 8 weeks Duration: 2.5 years |
|
| Participants | Inclusion: children up to 14 years of age operated for hydrocephalus of various aetiologies Exclusion: hypersensitivity to selected drug, patients who received antibiotic therapy in the period from 7 days before the operation, prophylactic antibiotic in the same period, concurrent infections, immunosuppressive therapy, diabetes and other factors that impair wound healing, prophylaxis inadvertently continued or another antibiotic administered within 10 days of operation. Any of the following diseases: agammaglobulinaemia, agranulocytosis, leukaemia, severe anaemia, lymphoma, thymus aplasia, melanoma, cirrhosis of the liver, burns or uremia Treatment: 50; control: 50 (66 ventriculoperitoneal shunts, 30 ventriculoatrial shunts and 4 duro‐peritoneal shunts) |
|
| Interventions | Intravenous prophylactic cefazedone (50 mg/kg) versus placebo | |
| Outcomes | Proportion of participants who had shunt infection 7 shunt infections occurred in the first 7 days and the other 3 were reported between the 2nd and 8th week |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "All patients with an even date of birth were allocated to active treatment group" |
| Allocation concealment (selection bias) | High risk | Birth dates can be checked |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Single blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Single blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available but published reports include all expected outcomes including analysis |
| Other bias | Unclear risk | The difference in outcome between the treatment group and control group seems substantial but it is not statistically significant. Other biases than the above described could contribute to this effect |
Bullock 1988.
| Methods | Randomized, controlled Method of randomisation: unclear Kind of shunt: ventriculoperitoneal shunts Location: South Africa Follow‐up: 90 days after surgery Duration: unclear |
|
| Participants | Inclusion: patients undergoing "clean" neurosurgical operative procedures Exclusion: patients with known piperacillin hypersensitivity or allergy, patients who had undergone a neurosurgical operation within a month prior to study and patients who had received any antibiotic therapy within 7 days prior to the study Treatment: 48, control 48 |
|
| Interventions | Piperacillin IV 35 mg/kg 30 to 60 minutes prior to surgery and 2 doses at 6 and 12 hours postoperatively Control: placebo (saline) |
|
| Outcomes | Sepsis (wound, meningitis) 3 cases of sepsis occurred within 22 days after surgery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "Allocation of antibiotic or placebo was carried out using a random number list" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Investigators remained blinded until the end of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 participants were excluded, exclusions were well described. A Chi² test was performed in order to check for statistically significant differences between exclusions in the treatment and placebo group. No statistical difference was found |
| Selective reporting (reporting bias) | Unclear risk | Not well described |
| Other bias | Unclear risk | 35 protocol deviations occurred, which could cause bias in outcome, even when statistical analysis both including and excluding these protocol deviations show no statistical differences |
Djindjian 1986.
| Methods | Randomisation, controlled Method of randomisation: unclear Kind of shunt: ventriculoperitoneal and ventriculoatrial shunts Location: France Follow‐up: minimally 6 months (for the most recent participants) Duration: 27 months |
|
| Participants | Inclusion: patients receiving their first implantation of shunts regardless of age Exclusion: antibiotics in the 14 days preceding surgery and surgery for replacement of a previously implanted shunt Treatment: 30; control: 30 |
|
| Interventions | Prophylactic oxacillin (200 mg/kg/day) for 24 hours perioperatively versus no antibiotics | |
| Outcomes | Proportion of participants who had CSF‐infection/meningitis (per age group). All infections occurred within 12 weeks after surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not directly described, but based on the workflow of administration of antibiotics and open aspect of the trial is assumed to be at high risk for allocation bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Open trial" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No apparent missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No detailed documentation |
| Other bias | Unclear risk | No other forms of bias could be directly detected. However, description of methods is limited; and based on the described study design it could be that other forms of bias are present |
Govender 2003.
| Methods | Randomisation, controlled Method of randomisation: unclear Kind of shunt: ventriculoperitoneal shunts Location: United Kingdom, multicentre Follow‐up: median of 9 months (range 1 to 20 months) Duration: unclear |
|
| Participants | Inclusion: patients with hydrocephalus who had been identified primarily as uninfected Exclusion: CSF infection, pregnant or lactating, known sensitivity to rifampin or clindamycin, harboured an open and uncorrected myelomeningocoele or encephalocoele Treatment: 50; control: 60 |
|
| Interventions | Ventriculoperitoneal shunt impregnated with clindamycin and rifampin (Bactiseal; Codman, Johnson & Johnson, Boston, MA) versus a control shunt | |
| Outcomes | Proportion of participants who had shunt infection Days to infection ranged from 6 days to 354 days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The difference in the colour of the shunts dictated the need for a blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15 participants (9.8%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study protocol well defined and pre‐specified outcome measures and analysis were available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Haines 1982.
| Methods | Randomised, controlled Method of randomisation: coin toss Kind of shunt: ventriculoperitoneal shunts Location: USA Follow‐up: 6 months Duration: unclear |
|
| Participants | Inclusion: children not allergic to penicillin and not on antibiotic therapy Exclusion: CSF infected at time of surgery or allergic reaction to methicillin Treatment: 35; control: 39 |
|
| Interventions | Methicillin IV (12.5mg/kg) every 6 hours beginning 6 hours before surgery and continuing for a total of 72 hours versus saline as placebo | |
| Outcomes | Proportion of participants who had shunt infection. 5 of the 7 infections occurred within the first month | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly selected by coin toss" |
| Allocation concealment (selection bias) | Low risk | Only the pharmacist had a record of the result of randomizations |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The drug and placebo were placed in identically marked vials |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants (4%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study protocol well defined and pre‐specified outcome measures and analysis were available |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lambert 1984.
| Methods | Randomised, controlled Method of randomisation: drawing a card Kind of shunt: ventriculoperitoneal and ventriculoatrial shunts Location: Germany Follow‐up: 6 months Duration: 21 months |
|
| Participants | Inclusion: any patient undergoing insertion or revision of a CSF shunt Exclusion: organisms resistant to gentamicin on surface swabs or recent surgery for CSF of shunt infection, antibiotics at time of surgery, revision of a colonized shunt system Treatment: 24; control: 20 |
|
| Interventions | Gentamicin IV/intramuscular (3 mg/kg) and gentamicin intrathecal 5 mg above the level of the revision either into the ventricle or the shunt system versus povidone iodine and a control group | |
| Outcomes | Proportion of participants who had shunt infection after new insertion or revision surgery No timing of outcome measurement described |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated to one of three groups by drawing a card" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Povidone iodine and antibiotic can be distinguished |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Povidone iodine and antibiotic can be distinguished |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exclusions not described |
| Selective reporting (reporting bias) | High risk | Study protocol not available and no pre‐specified outcome measures or analysis |
| Other bias | High risk | The dose of gentamicin was increased to 10 mg when ventricles were dilated. No cut‐off point for dilatation was described. No documentation about the number of participants with dilatated ventricles could be found |
Rieder 1987.
| Methods | Randomised, controlled Method of randomisation: draw system with sealed envelopes Type of shunt: ventriculoperitoneal shunts Location: London Follow‐up: 3 months Duration: 36 months |
|
| Participants | Inclusion: all children who presented for elective VP shunt insertion Exclusion: active infection or history of shunt infections. Immunosuppressed or receiving corticosteroid therapy. Allergy to penicillin or cephalothin Treatment: 32; control: 31 |
|
| Interventions | 25 mg/kg cephalothin IV before incision and 3 times postoperatively every 6 hours versus multivitamin preparation administration | |
| Outcomes | Proportion of participants who had shunt infection No clear timing of outcome measurement described |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Envelope draw system" |
| Allocation concealment (selection bias) | Low risk | "Pharmacist not otherwise involved in the patient's care" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The administered multivitamin sample in the control group has the same colour and is administered according to the same schedule as the cephalothin |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent missing outcome data and dropouts described |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcome measures were unavailable |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Schmidt 1985.
| Methods | Randomised, controlled Method of randomisation: random numbers Type of shunt: ventriculoperitoneal and ventriculoatrial shunts Location: Denmark Follow‐up: at least 6 months Duration: 18 months |
|
| Participants | Inclusion: a shunt or the revision of a shunt Exclusion: administration of antibiotics, signs of infection during the preceding 4 weeks. Stratification for age type of hydrocephalus, shunt type or number or type of previous operations was not done Treatment: 75, control: 73 |
|
| Interventions | 33.3 mg/kg methicillin given in 6 intravenous doses. The first dose was administered immediately after the induction and the last dose was given 20 hours later. Participants who were hypersensitive to penicillin received erythromycin. Control: no administration of antibiotics | |
| Outcomes | Proportion of participants who had shunt infection Onset of infection occurred within 7 months after surgery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were divided into 2 groups by random allocation by the use random numbers following Documenta Geige, Wissenschaftlichen Tabellen (reference 5 in the article) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Study was not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Study was not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent missing outcome data or dropouts |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Wang 1984.
| Methods | Randomised, controlled Method of randomisation: a table of random numbers Type of shunt: ventriculoperitoneal shunts Location: Canada Follow‐up: "until the last patient contact" Duration: 20 months |
|
| Participants | Inclusion: all patients that underwent ventriculoperitoneal shunt surgery at their hospital Exclusion: VPS infection within the preceding month. Received antibiotics within the previous week or patients who were known to be allergic to the intervention drug Treatment: 55; control: 65 |
|
| Interventions | 5 mg/kg trimethoprim and 25 mg/kg sulphamethoxazole IV. The first dose 1 hour before surgery. The second and third dose were given 8 and 16 hours after surgery. Control: dextrose solution given at the same time slot | |
| Outcomes | Proportion of participants who had shunt infection Mean days after surgery before onset of infection in the trimethoprim group was 24.5 days and 47 days in the sulphamethoxazole group |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 exclusions due to infection diagnosed perioperatively. Exclusions well defined |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available but it is clear that the published report includes all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
IV = intravenous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Eymann 2008 | A retrospective case‐control study. This was not clearly stated in the abstract |
| ISRCTN29451493 | They used external ventricular drains |
| Kumar 2016 | A prospective, observational study |
| Moussa 2017 | Antibiotic prophylaxis were used in the control group: single dose of cefotaxime 50mg/kg body weight given iv. within 60min prior to skin incision repeated once postoperatively |
| NCT00280904 | Observational study |
| NCT00286104 | They used external ventricular drains |
| NCT01936272 | Ventricularperitoneal shunt placement versus endoscopic third ventriculostomy/choroid plexus cauterization |
| NCT02600793 | It is non‐randomised and they used external ventricular drains |
| Nejat 2008 | Trimethoprim‐sulfamethoxazole 5mg/kg was used in the control group |
| Odio 1984 | Unclear what shunt type was used |
| Tacconelli 2008 | This study did not report on shunt infections in internal shunts |
| Theophilus 2011 | The control group consisted of regular care with a single prophylactic dose of iv cefuroxime 25mg/kg body weight |
| Walters 1992 | Not described what shunt type was used |
| Young 1987 | Not described what shunts are used |
| Zentner 1995 | This study did not report on shunt infections in internal shunts |
Differences between protocol and review
The primary and was reformulated and the subgroup "Difference in infection rate between ventriculoperitoneal and ventriculoatrial shunts" was added.
Contributions of authors
Draft the protocol ‐ Sebastian Arts
Develop and run the search strategy ‒ Cochrane Multiple Sclerosis and Rare Diseases of the CNS Review Group
Obtain copies of studies ‒ Sebastian Arts
Select which studies to include ‒ all authors
Extract data from studies ‒ Sebastian Arts and Erik van Lindert
Enter data into RevMan ‒ Sebastian Arts
Carry out the analysis ‒ Sebastian Arts and Hieronymus Boogaarts
Interpret the analysis ‒ all authors
Draft the final review ‒ Sebastian Arts
Update the review ‒ all authors
Sources of support
Internal sources
-
Radboud University Nijmegen, Netherlands.
- Librarian connected to the library of medical sciences at the Radboud University Medical Center has helped to set‐up a search strategy
External sources
No sources of support supplied
Declarations of interest
SA ‒ none
HB ‒ none
EvL ‒ none
New
References
References to studies included in this review
Bayston 1990 {published data only}
- Bayston R, Bannister C, Boston V, Burman R, Burns B, Cooke F, et al. A prospective randomised controlled trial of antimicrobial prophylaxis in hydrocephalus shunt surgery. Zeitschrift fur Kinderchirurgie [Surgery in Infancy and Childhood] 1991;45(Suppl 1):5‐7. [DOI] [PubMed] [Google Scholar]
Blomstedt 1985 {published data only}
- Blomstedt GC. Results of trimethoprim‐sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures: A double‐blind randomized trial. Journal of Neurosurgery 1985;62(5):694‐7. [DOI] [PubMed] [Google Scholar]
Blum 1989 {published data only}
- Blum J, Schwarz M, Voth D. Antibiotic single‐dose prophylaxis of shunt infections. Neurosurgical Review 1989;12(3):239‐44. [DOI] [PubMed] [Google Scholar]
Bullock 1988 {published data only}
- Bullock R, Dellen JR, Ketelbey W, Reinach SG. A double‐blind placebo‐controlled trial of perioperative prophylactic antibiotics for elective neurosurgery. Journal of Neurosurgery 1988;69(5):687‐91. [DOI] [PubMed] [Google Scholar]
Djindjian 1986 {published data only}
- Djindjian M, Fevrier M, Otterbein G, Soussy J. Oxacillin prophylaxis in cerebrospinal fluid shunt procedures: results of a randomized open study in 60 hydrocephalic patients. Surgical Neurology 1986;25(2):178‐80. [DOI] [PubMed] [Google Scholar]
Govender 2003 {published data only}
- Govender ST, Nathoo N, Dellen JR. Evaluation of an antibiotic‐impregnated shunt system for the treatment of hydrocephalus. Journal of Neurosurgery 2003;99(5):831‐9. [DOI] [PubMed] [Google Scholar]
Haines 1982 {published data only}
- Haines SJ, Taylor F. Prophylactic methicillin for shunt operations: effects on incidence of shunt malfunction and infection. Child's Brain 1982;9(1):10‐22. [DOI] [PubMed] [Google Scholar]
Lambert 1984 {published data only}
- Lambert M, MacKinnon AE, Vaishnav A. Comparison of two methods of prophylaxis against CSF shunt infection. Zeitschrift fur Kinderchirurgie [Surgery in Infancy and Childhood] 1984;39(Suppl 2):109‐10. [DOI] [PubMed] [Google Scholar]
Rieder 1987 {published data only}
- Rieder MJ, Frewen TC, Maestro RF, Coyle A, Lovell S. The effect of cephalothin prophylaxis on postoperative ventriculoperitoneal shunt infections. CMAJ : Canadian Medical Association Journal 1987;136(9):935‐8. [PMC free article] [PubMed] [Google Scholar]
Schmidt 1985 {published data only}
- Schmidt K, Gjerris F, Osgaard O, Hvidberg EF, Kristiansen JE, Dahlerup B, et al. Antibiotic prophylaxis in cerebrospinal fluid shunting: a prospective randomized trial in 152 hydrocephalic patients. Neurosurgery 1985;17(1):1‐5. [DOI] [PubMed] [Google Scholar]
Wang 1984 {published data only}
- Wang EE, Prober CG, Hendrick BE, Hoffman HJ, Humphreys RP. Prophylactic sulfamethoxazole and trimethoprim in ventriculoperitoneal shunt surgery A double‐blind, randomized, placebo‐controlled trial. JAMA 1984;251(9):1174‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Eymann 2008 {published data only}
- Eymann R, Chehab S, Strowitzki M, Steudel W, Kiefer M. Clinical and economic consequences of antibiotic‐impregnated cerebrospinal fluid shunt catheters. Journal of Neurosurgery. Pediatrics 2008;1(6):444‐50. [DOI] [PubMed] [Google Scholar]
ISRCTN29451493 {published data only}
- ISRCTN29451493. Silver impregnated line versus external ventricular drains (EVD) randomised trial [Silver impregnated line versus EVD randomised trial and cerebrospinal fluid infection from the use of external ventricular drains]. www.isrctn.com/ISRCTN29451493 (first received 12 September 2015).
Kumar 2016 {published data only}
- Kumar R, Younus SM, Haseeb A, Bilal M, Zeeshan QM, Ashraf J. Frequency of infection associated with ventriculo‐peritoneal shunt placement. Journal of the Pakistan Medical Association 2016;66(7):815‐8. [PubMed] [Google Scholar]
Moussa 2017 {published data only}
- Moussa WM, Mohamed MA. Efficacy of postoperative antibiotic injection in and around ventriculoperitoneal shunt in reduction of shunt infection a randomized controlled trial. Clinical Neurology and Neurosurgery 2017;143:144‐9. [DOI] [PubMed] [Google Scholar]
NCT00280904 {published data only}
- NCT00280904. A registry for comparing catheter‐related infection rates among various shunt systems in the treatment of hydrocephalus [A Registry for Comparing Catheter‐Related Infection Rates Among Various Shunt Systems in the Treatment of Hydrocephalus]. clinicaltrials.gov/ct2/show/NCT00280904 (first received 24 January 2006).
NCT00286104 {published data only}
- NCT00286104. Impact of ventricular catheter used with antimicrobial agents on patients with a ventricular catheter [The impact of ventricular catheter impregnated with antimicrobial agents on infection in patients with ventricular catheter: a prospective randomized study]. clinicaltrials.gov/ct2/show/NCT00286104 (first received 3 February 2006).
NCT01936272 {published data only}
- NCT01936272. Randomized controlled trial of shunt vs ETV/CPC for PIH in Ugandan infants [Neurocognitive outcomes and changes in brain and cerebral spinal fluid (CSF) volume after treatment of post‐infectious hydrocephalus (PIH) in Ugandan infants by shunting versus ETV/CPC]. clinicaltrials.gov/ct2/show/NCT01936272 (first received 6 September 2013).
NCT02600793 {published data only}
- NCT02600793. Ceftaroline diffusion into cerebrospinal fluid of children [Ceftaroline diffusion into cerebrospinal fluid of children with ventriculitis due to ventriculoperitoneal shunt (VPS) infection]. clinicaltrials.gov/ct2/show/NCT02600793 (first received 9 November 2015).
Nejat 2008 {published data only}
- Nejat F, Tajik P, Khashab M, Kazmi SS, Khotaei GT, Salahesh S. A randomized trial of ceftriaxone versus trimethoprim‐sulfamethoxazole to prevent ventriculoperitoneal shunt infection. Journal of Microbiology, Immunology and Infection 2008;41(2):112‐7. [PubMed] [Google Scholar]
Odio 1984 {published data only}
- Odio C, Mohs E, Sklar FH, Nelson JD, McCracken GH. Adverse reactions to vancomycin used as prophylaxis for CSF shunt procedures. American Journal of Diseases of Children (1960) 1984;138(1):17‐9. [DOI] [PubMed] [Google Scholar]
Tacconelli 2008 {published data only}
- Tacconelli E, Cataldo MA, Albanese A, Tumbarello M, Arduini E, Spanu T, et al. Vancomycin versus cefazolin prophylaxis for cerebrospinal shunt placement in a hospital with a high prevalence of meticillin‐resistant Staphylococcus aureus. Journal of Hospital Infection 2008;69(4):337‐44. [DOI] [PubMed] [Google Scholar]
Theophilus 2011 {published data only}
- Theophilus SC, Adnan SJ. A randomised control trial on the use of topical methicillin in reducing post‐operative ventriculoperitoneal shunt infection. Malaysian Journal of Medical Sciences 2011;18(1):30‐7. [PMC free article] [PubMed] [Google Scholar]
Walters 1992 {published data only}
- Walters BC, Goumnerova L, Hoffman HJ, Hendrick EB, Humphreys RP, Levinton C. A randomized controlled trial of perioperative rifampin/trimethoprim in cerebrospinal fluid shunt surgery. Child's Nervous System : ChNS : Official Journal of the International Society for Pediatric Neurosurgery 1992;8(5):253‐7. [DOI] [PubMed] [Google Scholar]
Young 1987 {published data only}
- Young RF, Lawner PM. Perioperative antibiotic prophylaxis for prevention of postoperative neurosurgical infections. A randomized clinical trial. Journal of Neurosurgery 1987;66(5):701‐5. [DOI] [PubMed] [Google Scholar]
Zentner 1995 {published data only}
- Zentner J, Gilsbach J, Felder T. Antibiotic prophylaxis in cerebrospinal fluid shunting: a prospective randomized trial in 129 patients. Neurosurgical Review 1995;18(3):169‐72. [DOI] [PubMed] [Google Scholar]
Additional references
Attenello 2010
- Attenello FJ, Garces‐Ambrossi GL, Zaidi HA, Sciubba DM, Jallo GI. Hospital costs associated with shunt infections in patients receiving antibiotic‐impregnated shunt catheters versus standard shunt catheters. Neurosurgery 2010;66(2):284‐9. [DOI] [PubMed] [Google Scholar]
Bondurant 1995
- Bondurant CP, Jimenez DF. Epidemiology of cerebrospinal fluid shunting. Pediatric Neurosurgery 1995;23(5):254‐8. [DOI] [PubMed] [Google Scholar]
Borgbjerg 1995
- Borgbjerg BM, Gjerris F, Albeck MJ, Borgesen SE. Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first‐time shunts. Acta Neurochirurgica 1995;136(1‐2):1‐7. [DOI] [PubMed] [Google Scholar]
Casey 1997
- Casey AT, Kimmings EJ, Kleinlugtebeld AD, Taylor WA, Harkness WF, Hayward RD. The long‐term outlook for hydrocephalus in childhood. A ten‐year cohort study of 155 patients. Pediatric Neurosurgery 1997;27(2):63‐70. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Drake 1998
- Drake JM, Kestle JR, Milner R, Cinalli G, Boop F, Piatt J, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery 1998;43(2):294‐303. [DOI] [PubMed] [Google Scholar]
Frame 1984
- Frame PT, McLaurin RL. Treatment of CSF shunt infections with intrashunt plus oral antibiotic therapy. Journal of Neurosurgery 1984;60(2):354‐60. [DOI] [PubMed] [Google Scholar]
Fu 2002
- Fu L, Tang Y, Wang S. Effect of nonoperative treatment on the outcome of patients with posttraumatic hydrocephalus. Chinese Journal of Traumatology 2002;5(1):7‐11. [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version 3.6. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Greenberg 2010
- Greenberg MS. Shunt infection. Handbook of Neurosurgery. 7th Edition. New York: Thieme, 2010:345‐8. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. Rating quality of evidence and stregth ofrecommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hebb 2001
- Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery 2001;49(5):1166‐84. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Green S, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
James 2014
- James G, Hartley JC, Morgan RD, Ternier J. Effect of introduction of antibiotic‐impregnated shunt catheters on cerebrospinal fluid shunt infection in children: a large single‐center retrospective study. Journal of Neurosurgery. Pediatrics 2014;13(1):101‐6. [DOI] [PubMed] [Google Scholar]
Kestle 2011
- Kestle JR, Riva‐Cambrin J, Wellons JC 3rd, Kulkarni AV, Whitehead WE, Walker ML, et al. A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. Journal of Neurosurgery. Pediatrics 2011;8(1):22‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kestle 2016
- Kestle JR, Holubkov R, Cochrane D, Kulkarni AV, Limbrick DD, Luerssen TG, et al. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. Journal of Neurosurgery. Pediatrics 2016;17(4):391‐6. [DOI] [PubMed] [Google Scholar]
Klimo 2014
- Klimo P, Poppel M, Thompson CJ, Baird LC, Duhaime AC, Flannery AM, et al. Pediatric hydrocephalus: systematic literature review and evidence‐based guidelines. Part 6: Preoperative antibiotics for shunt surgery in children with hydrocephalus: a systematic review and meta‐analysis. Journal of Neurosurgery. Pediatrics 2014;14(Suppl 1):44‐52. [DOI] [PubMed] [Google Scholar]
Konstantelias 2015
- Konstantelias AA, Vardakas KZ, Polyzos KA, Tansarli GS, Falagas ME. Antimicrobial‐impregnated and ‐coated shunt catheters for prevention of infections in patients with hydrocephalus: a systematic review and meta‐analysis. Journal of Neurosurgery 2015;122(5):1096‐112. [DOI] [PubMed] [Google Scholar]
Lefevbre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Limbrick 2014
- Limbrick DD, Baird LC, Klimo P, Riva‐Cambrin J, Flannery AM. Pediatric hydrocephalus: systematic literature review and evidence‐based guidelines. Part 4: Cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. Journal of Neurosurgery. Pediatrics 2014;14(Suppl 1):30‐4. [DOI] [PubMed] [Google Scholar]
Moussa 2016
- Moussa WM, Mohamed MA. Efficacy of postoperative antibiotic injection in and around ventriculoperitoneal shunt in reduction of shunt infection: A randomized controlled trial. Clinical Neurology and Neurosurgery 2016;143:144‐9. [DOI] [PubMed] [Google Scholar]
Ragel 2006
- Ragel BT, Browd SR, Schmidt RH. Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. Journal of Neurosurgery 2006;105(2):242‐7. [DOI] [PubMed] [Google Scholar]
Ratilal 2008
- Ratilal B, Costa J, Sampaio C. Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts: a systematic review. Journal of Neurosurgery. Pediatrics 2008;1(1):48‐56. [DOI] [PubMed] [Google Scholar]
Rekate 1999
- Rekate HL. Treatment of hydrocephalus. In: Albright AL, Pollack IF, Adelson PD editor(s). Principles and Practice of Pediatric Neurosurgery. New York: Thieme, 1999:47‐75. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sciubba 2007
- Sciubba DM, Lin LM, Woodworth GF, McGirt MJ, Carson B, Jallo GI. Factors contributing to the medical costs of cerebrospinal fluid shunt infection treatment in pediatric patients with standard shunt components compared with those in patients with antibiotic impregnated components. Neurosurgical Focus 2007;22(4):E9. [DOI] [PubMed] [Google Scholar]
Simon 2009
- Simon TD, Hall M, Riva‐Cambrin J, Albert JE, Jeffries HE, Lafleur B, et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. Journal of Neurosurgery. Pediatrics 2009;4(2):156‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2010
- Simon TD, Hall M, Dean JM, Kestle JR, Riva‐Cambrin J. Reinfection following initial cerebrospinal fluid shunt infection. Journal of Neurosurgery. Pediatrics 2010;6(3):277‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Lindert 2018
- Lindert EJ, Bilsen MV, Flier MV, Kolwijck E, Delye H, Oever JT. Topical vancomycin reduces the cerebrospinal fluid shunt infection rate: A retrospective cohort study. PLoS One 2018;13(1):e0190249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yogev 1985
- Yogev R. Cerebrospinal fluid shunt infections: a personal view. Pediatric Infectious Disease 1985;4(2):113‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Arts SHHMJ at al, 2017
- Arts SHHMJ, Boogaarts HD, Lindert EJ. Route of antibiotic prophylaxis for prevention of cerebrospinal fluid‐shunt infection. Cochrane Database of Systematic Reviews 2017, Issue 12. [DOI: 10.1002/14651858.CD012902] [DOI] [PMC free article] [PubMed] [Google Scholar]


