Scheme 2.
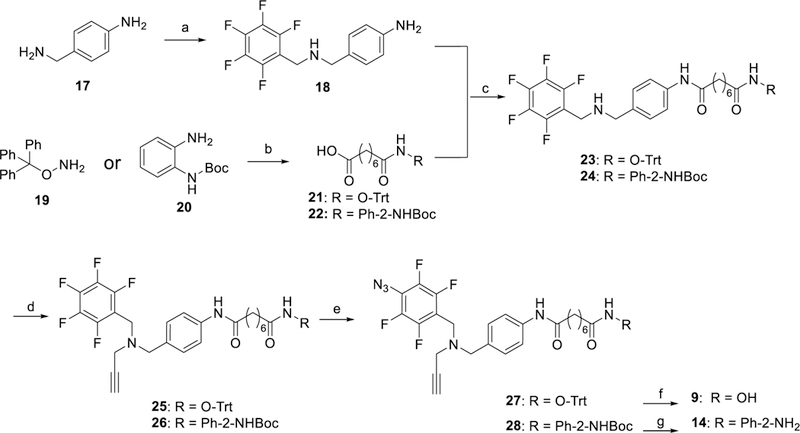
Synthesis of PRPs 9 and 14. Reagents and Conditions: a) 1. perfluorobenzaldehyde, DCM,18 h, 2. NaBH4, CH3OH, 0 °C→rt, 4 h; b) 1. octanedioic acid monomethyl ester, EDC•HCl, HOBt, DMAP, Et3N, CHCl3, 18 h, 2. NaOH, 2:1 MeOH/H2O, 8 h; c) EDC•HCl, HOBt, Et3N, CHCl3, 12 h; d) propargyl bromide, K2CO3, CH3CN, 16 h; e) NaN3, Bu4NN3, DMF, 75–80 °C, 18 h; f) MgBr2, DCM, 30 min; g) HCl, 1,4-dioxane, 0 °C, 3 h.
