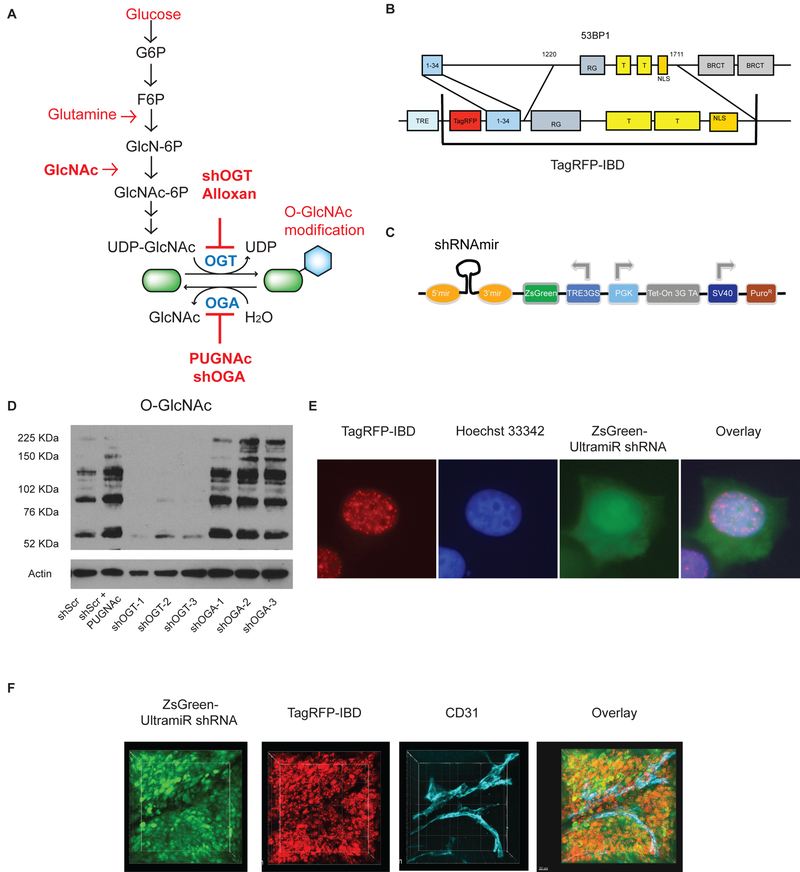
Figure 1.
Tools to target protein O-GlcNAcylation and track impacts on DNA damage response in vitro and in vivo. A, The rate limiting step of the hexosamine biosynthetic pathway (HBP) is the transfer of an amine from glutamine to glucose as fructose 6-phosphate (F6P) to form glucosamine 6-phosphate (GlcN-6P) and glutamate. Once acetylated, N-acetylglucosamine 6-phosphate (GlcNAc-6P) is linked to uridine diphosphate to form UDP-GlcNAc. Exogenous GlcNAc is readily phosphorylated and can drive the pathway without feeding glycolysis or glutaminolysis. Although UDP-GlcNAc is often considered as a key metabolite for secretory glycosylation, the enzyme O-GlcNAc transferase (OGT) modifies intracellular protein serines and threonines with the O-GlcNAc post-translational modification. Typically, O-GlcNAcase (OGA) rapidly removes the GlcNAc, restoring the hydroxyl form. Alloxan and PUGNAc are small molecule OGT and OGA inhibitors, respectively. B, The TagRFP-IBD reporter expresses TagRFP fused to residues 1–34 and 1220–1711 of human 53BP1. C, The ZsGreen-shRNA-miR reporter construct for silencing OGT or OGA. D, Western blot analysis of the O-GlcNAc protein modification in MCF7TagRFP-IBD cell lines with shRNAs targeting OGT (shOGT) or OGA (shOGA). Three gene-specific shRNA-miRs were tested for each target. Random scrambled control shRNA-miR (shScr) and the OGA inhibitor PUGNAc were used as negative and positive controls, respectively, for O-GlcNAc protein modification. E, Representative cell image with TagRFP-IBD and ZsGreen-shRNA-miR (Scr) fluorescence examined 2 h after irradiation with 6 Gy (TagRFP, red; ZsGreen, green; Hoechst 33342, blue). F, Intratumoral imaging of MCF7 cells with dual fluorescent reporters for IRIF (TagRFP, red) and shRNA-miR expression (ZsGreen, green) after irradiation with 6 Gy. Anti-CD31 antibody was used for vascular staining (endothelium, cyan).