Figure 1. VEGF/VEGFR signaling promotes angiogenesis and suppresses antitumor immunity.
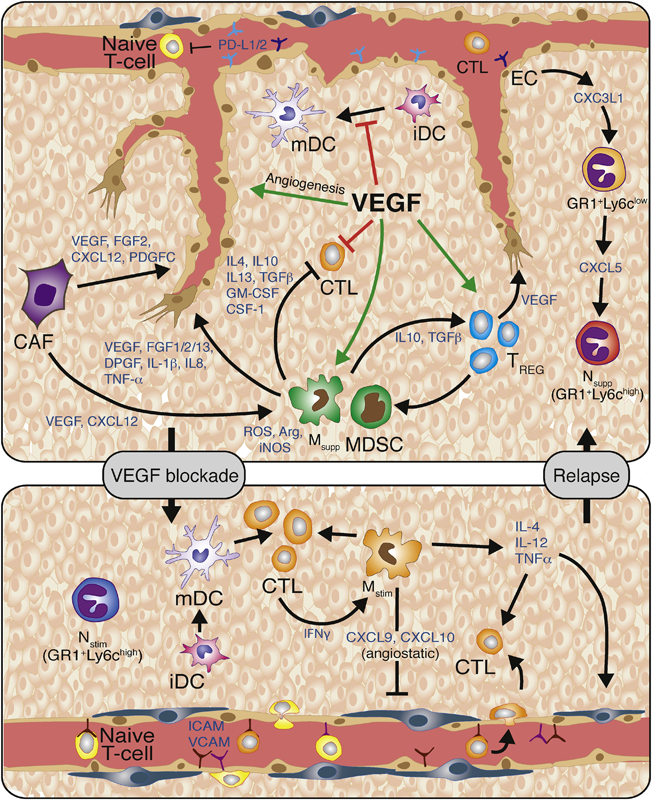
VEGF promotes endothelial cell proliferation, migration and survival. VEGF also promotes immunosuppression by inducing endothelial cell expression of the PD1 ligands PD-L1 and PD-L2 that interact with PD-1 on T-cells resulting in reduced T-cell proliferation and effector function. VEGF also inhibits T-cell-adhesion to the luminal surface of the vasculature and subsequent extravasation into the tumor by blocking TNFα-induced expression of VCAM and ICAM. VEGF can also directly block dendritic cell function by inhibiting dendritic cell maturation and inducing PD-L1 expression on mature dendritic cells. It inhibits the proliferation and effector function of cytotoxic T-cells, while inducing regulatory T-cell (Treg) proliferation. Tregs secrete various cytokines and growth factors, including IL-10, IL-4, IL-13, TGFβ1, GM-CSF, and CSF-1, which recruit angiogenic and immune-suppressive MDSC and TAMs. MDSC and TAMs then produce reactive oxygen species, nitric oxide, and arginase to suppress T-cell proliferation, viability, and activity. Thus, inhibition of VEGF should restore many of these phenotypes. Congruently, VEGF-blockade enables the endothelium to facilitate T-cell infiltration due to vessel normalization accompanied with elevated levels of I-CAM and V-CAM lymphocyte adhesion molecules. Furthermore, VEGF inhibition enables dendritic cell maturation and function, and thus increases levels of intratumoral effector T-cells. Anti-VEGF therapy also increases the presence of Th1/M1-immunosupportive/angiostatic myeloid cells (e.g; macrophages, neutrophils). Subsequently, VEGF-blockade should unleash the anti-tumor immune response and lead to increased tumor cell apoptosis. Further vessel pruning, however, creates hypoxic areas that drive the recruitment and polarization of immunosuppressive and angiogenic innate immune cells. Therefore, therapeutic approaches that promote immunosurveillance, can enhance and sustain the efficacy of antiangiogenic therapy. Abbreviations: VEGF, vascular endothelial growth factor; PDL1/2, programmed death ligand 1/2; VCAM, vascular cell adhesion molecule; ICAM, intercellular adhesion molecule; iDC, immature dendritic cell; DC, dendritic cell; CTL, cytotoxic T-cell; Treg, regulatory T-cell; ROS, reactive oxygen species; NO, nitric oxide; Arg1, arginase-1; IL-10, -4, -13, -12, - 23, -1b, -8, interleukin-10, -4, -13, -12, -23, -1b, -8; TGFβ1, transforming growth factor-β1; GM-CSF, granulocyte/monocyte-colony stimulating factor; CSF-1, colony stimulating factor-1; G- or M- MDSC, granulocytic or monocytic-myeloid derived suppressor cell; M2- TAM, M2-polarized macrophage; FGF-1, -2, -13, fibroblast growth factor-1, -2, -13; PDGF, platelet-derived growth factor; TNFα, tumor necrosis factor-α NK-cell, natural killer cell; Th1, T-helper 1.
