Figure EV2. cTAZ was not regulated by Hippo signaling.
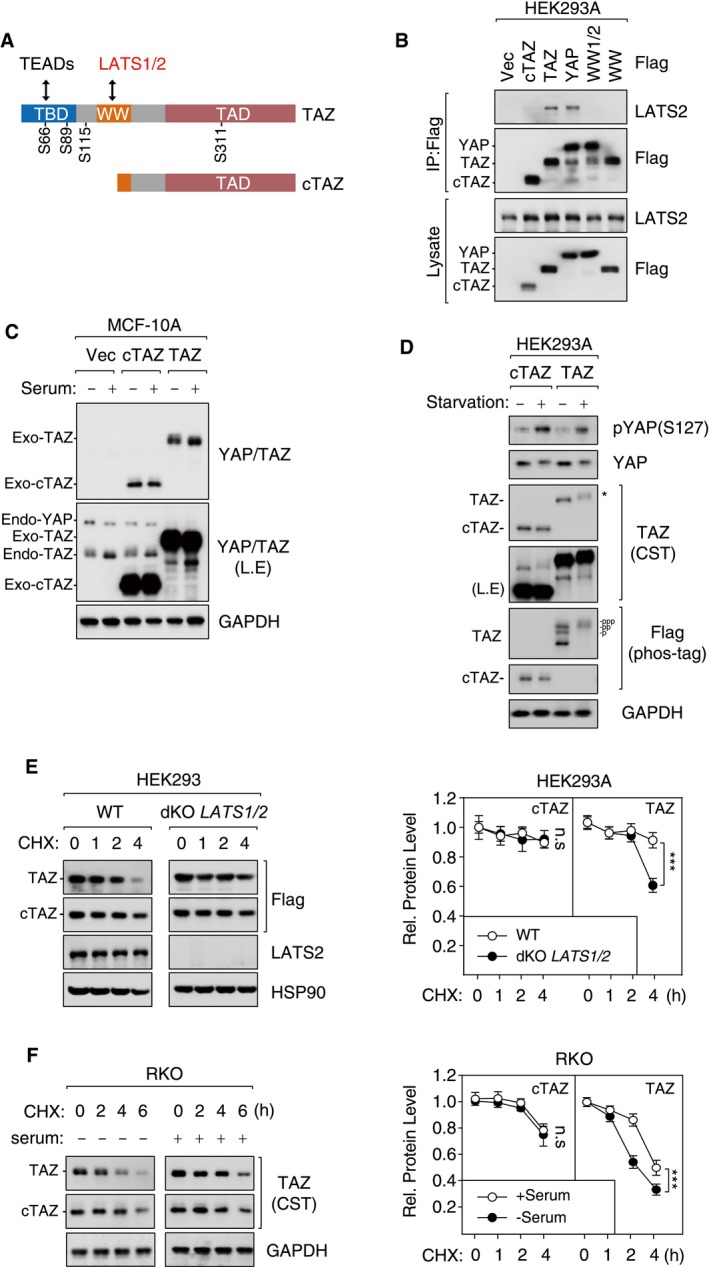
- Protein domains and interacting proteins of TAZ. Phosphorylation sites of LATS kinases are shown.
- cTAZ did not interact with LATS2. HEK293A cells were transfected with Flag‐tagged cTAZ, TAZ, YAP, WW‐mutated TAZ (WW), and WW1/2‐mutated YAP (WW1/2), and cell lysates were prepared and used for IP.
- cTAZ was not regulated by serum stimulation. cTAZ‐ or TAZ‐overexpressing MCF‐10A cells were stimulated by 10% FBS for 1 h, and protein expression was determined. Ectopic and endogenous TAZ were down‐shifted upon serum stimulation, whereas no change for cTAZ was detected.
- cTAZ was not regulated by serum starvation. cTAZ‐ or TAZ‐overexpressing HEK293A cells were serum‐starved for 8 h and harvested for IB. The phosphorylation of full‐length TAZ was also determined using phos‐tag gel. The asterisk indicates a shift of TAZ on regular gel.
- Deletion of LATS kinases stabilized TAZ, but not cTAZ. WT or LATS1/2‐double‐knockout (dKO) HEK293A cells were transfected with Flag‐tagged cTAZ or TAZ. Cells were treated with CHX (50 μg/ml) in a time course (0–4 h), and protein levels were determined. Error bars indicate SD, n = 3. ***P < 0.001; two‐way ANOVA test was used for statistical analysis.
- Serum treatment stabilized TAZ, but not cTAZ. RKO cells cultured in the presence or absence of 10% serum were treated with CHX (50 μg/ml) in a time course (0–6 h), and protein levels were determined. Error bars indicate SD, n = 3. ***P < 0.001; two‐way ANOVA test was used for statistical analysis.
Source data are available online for this figure.
