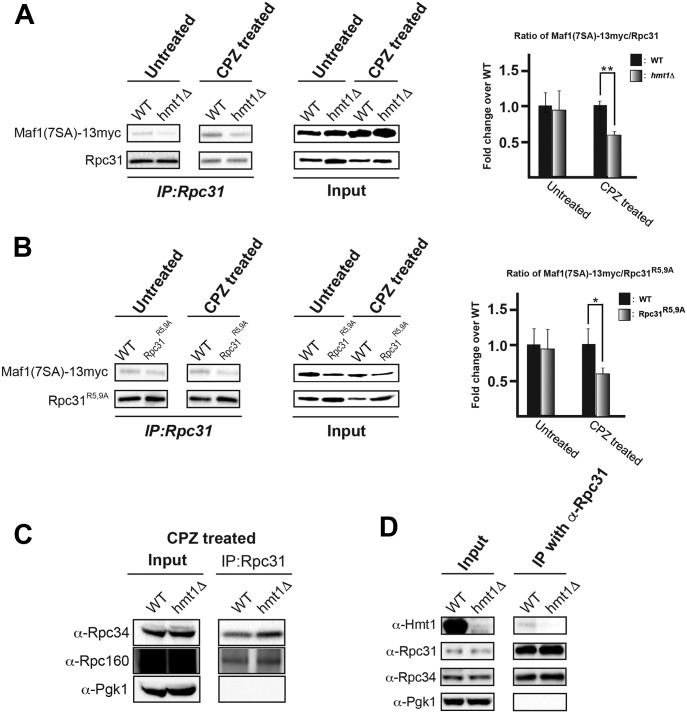
Figure 5. During stress, methylation of Rpc31 is required for the association of Pol III with its negative regulator Maf1.
(A) Rpc31 levels in yeast lysates generated from WT or hmt1Δ cells before and after treatment with CPZ, assessed by CoIP with an α-Rpc31 antibody. The levels of Myc-tagged Maf1-7SA were probed using an α-Myc antibody and the results are displayed in a bar graph. Error bars represent the SEM of three biological replicates (n = 3). P-value as calculated by t test: *<0.05 and **<0.01. (B) Rpc31 and Myc-tagged Maf1-7SA levels in yeast lysates generated from WT or Rpc31R5,9A cells before and after treatment of CPZ, assessed using CoIP as in (A) (including the number of replicates). P-value as calculated by t test: *<0.05 and **<0.01. (C) Rpc160 and Rpc34 levels in complexes immunoprecipitated with α-Rpc31 antibody. The samples were probed with α-Rpc160, α-Rpc34, and α-Pgk1 (as a negative control). (D) Hmt1 physically interacts with Rpc31-containing complex. CoIP of Rpc31 was carried out using cell lysates generated from WT or hmt1Δ cells and resolved on a 4–12% SDS–PAGE followed by immunoblotting using α-Hmt1, α-Rpc31, and α-Rpc34 antibodies to determine the levels of Hmt1, Rpc31, and Rpc34 present in the co-immunoprecipitates. The level of Pgk1 was used as a negative control in the immunoprecipitation experiment.
Source data are available for this figure.