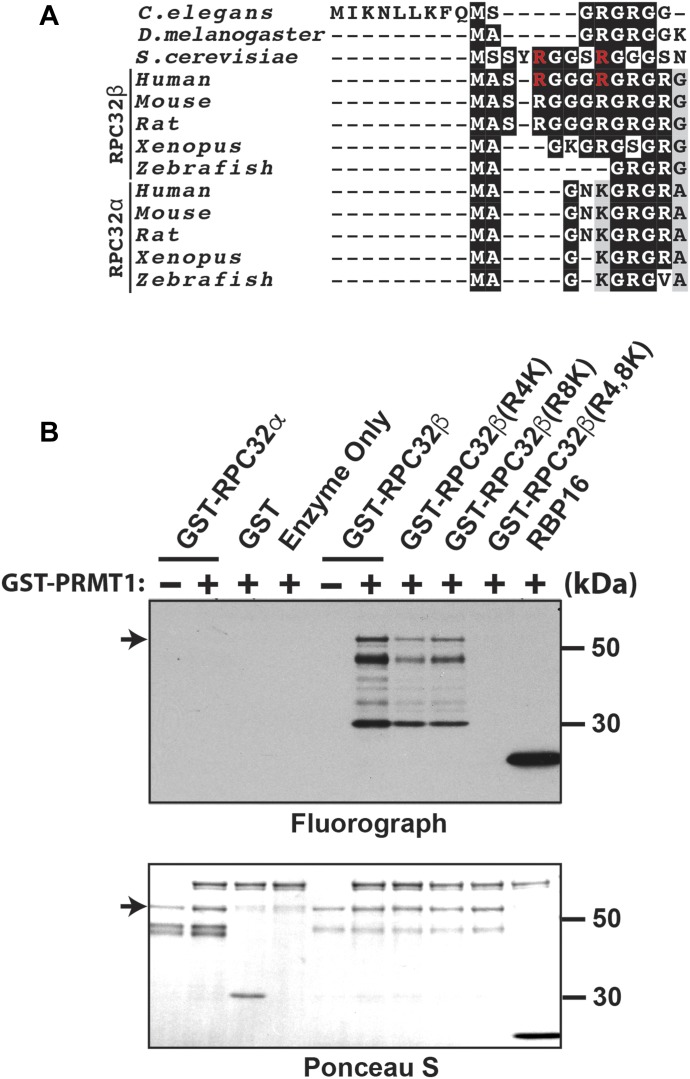
Figure 7. Mammalian PRMT1 methylates human Rpc31 homolog RPC32β, but not RPC32α.
(A) Sequence alignment of N-terminal amino acid sequences of yeast Rpc31 with its homologs in Caenorhabditis elegans, Drosophila melanogaster, and various mammalian species. Arginines on yeast Rpc31 that are colored red represent the identified methylated arginine and are conserved in RPC32β. (B) WT RPC32α and RPC32β were purified from E. coli and then subjected to in vitro methylation by rat PRMT1 and [methyl-3H]-SAM. In parallel, methylarginine substitutionmutants of RPC32β (R4K, R8K and R4, 8K) were also tested. The arrow on the fluorograph denotes methylated RPC32β. RBP16 served as a positive control for the in vitro methylation. The protein loading levels for each sample areshownby Ponceau S staining of the same membrane before fluorography, with the arrow denoting the substrate tested.