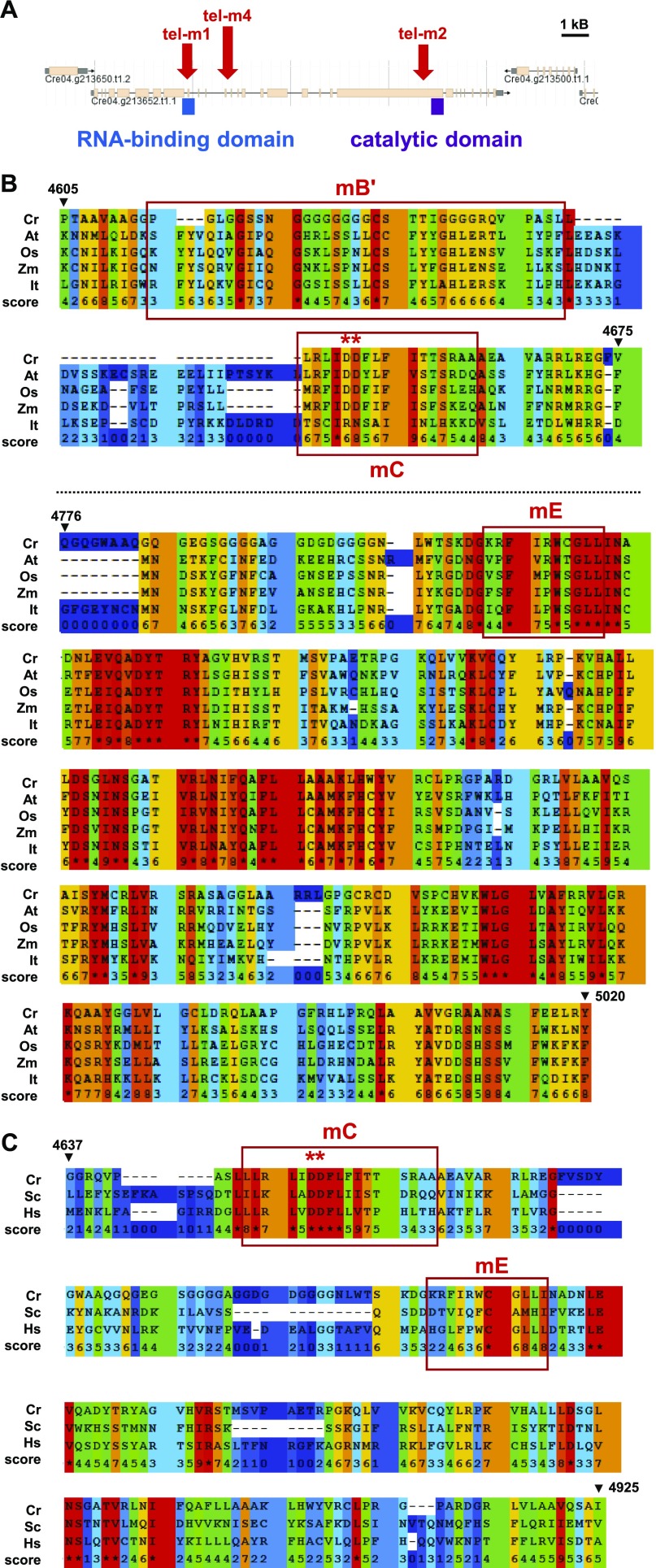
Figure 4. Identification of the CrTERT gene encoding the catalytic subunit of telomerase in C. reinhardtii.
(A) The protein corresponding to the predicted gene model Cre04.g213652.t1.1 of the available C. reinhardtii nuclear genome harbors an annotated N-terminal domain with significant similarities to the RNA template-binding domain of telomerases from other organisms. The C-terminal domain shows strong similarities with the catalytic domain of this enzyme in other organisms. Mutants tel-m1 (LMJ.RY0402.077111) and tel-m2 (LMJ.RY0402.209904) from the CliP library have reported insertions in either the RNA-binding or the catalytic domain, respectively. Mutant tel-m4 (LMJ.RY0402.105594) has an insertion in between these two domains. (B) PSI-blast alignments show strong amino-acid sequence similarity of thecatalytic domain of telomerases from many organisms with the putative C. reinhardtii protein. Similarity score ranges from 0 (light blue) to 9 and * (red) indicates identity. The motifs B′, C, and E (mB′, mC, and mE) described by Lingner et al (1997b), Ogushi et al (1999) show strong conservation in C. reinhardtii, including two catalytic aspartates, essential for telomerase function in other organisms (red asterisks). Conservation can also be observed downstream of mE between CrTERT and the other telomerases. (C) The mC motif of C. reinhardtii shows strong sequence similarity with the mC motif containing two catalytically essential aspartates in yeast and human telomerases (Lingner et al, 1997b; Ogushi et al, 1999). Cr, C. reinhardtii; At, A. thaliana; Os, O. sativa; Zm, Z. mays; lt, I. tectorum; Sc, S. cerevisiae; Hs, H. sapiens.