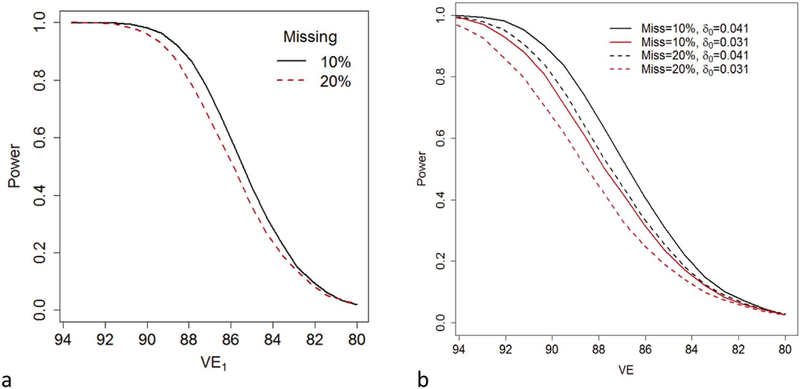
Fig. 1.
(a) The power to test the null hypothesis that the difference, Δ = π1 − π2, in incident infection rates between a 1-dose and 2-dose arm exceeds 0.00987 as a function of the vaccine efficacy, VE1, of the 1-dose regimen with a one-sided α = 0.025. Each arm of the trial initially contains 5000 individuals. Among the 5000 girls in each arm, either 10% of girls are lost to follow-up and 10% of visits are not attended (Missing = 10%; black/solid) or 20% of girls are lost to follow-up and 20% of visits are not attended (Missing = 20%; red/dashed). We assumed that the probability, π0, that a trial participant would acquire an incident persistent infection is 0.0725 had they not been vaccinated and that vaccine efficacy of the 2-dose regimen is 93.6%. (b) The power to reject the null hypothesis VE < 80% as a function of the VE of the tested regimen at a one-sided α = 0.025. We assume that the survey arm and trial arm start with 5000 individuals. We assume that the risk of an incident infection, in the absence of vaccination, spanning the last two trial visits would be either δ0 = 0.041 (black) or δ0 = 0.031 (red). Among the 5000 girls in the trial arm, either 10% of girls are lost to follow-up and 10% (among those girls not lost to follow-up) of each of the final two visits are not attended (Miss = 10%; solid) or 20% of girls are lost to follow-up and 20% of visits are not attended (Miss = 20%; dashed). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)