Abstract
Background: To evaluate the effects of resveratrol to monocyte chemoattractant protein-1 (MCP-1) and the role of p38 mitogen-activated protein kinase (MAPK) in this process in vitro.
Materials and methods: Animal acute pulmonary thromboembolism (PTE) model: rat model was established by infusion of an autologous blood clot into the pulmonary artery through a polyethylene catheter. One hundred and thirty-two rats were randomly and equally divided into ten groups: rats-control (untreated), rats-1% DMSO, rats-TNF-α, rats-TNF-α + resveratrol, rats-TNF-α +C1142, rats-TNF-α+SB203580, rats-TNF-α+resveratrol + SB203580, rats-resveratrol only, rats-C1142 only, and rats-SB203580 only. Rat pulmonary artery endothelial cells (RPAs) tests: RPAs were isolated from above animal and designated as: RPAs-control, RPAs-1% DMSO control, RPAs-TNF-α, RPAs-TNF-α + resveratrol, RPAs-TNF-α + C1142, RPAs-TNF-α + SB203580, RPAs-TNF-α + resveratrol + SB203580, RPAs-resveratrol only, RPAs-C1142 only, and RPAs-SB203580 only. Each group was further divided into 1, 4, and 8 hrs time point for evaluation (n=6 rats per time point) except RPAs-TNF-α + SB203580, RPAs-TNF-α + resveratrol + SB203580, RPAs-C1142 and RPAs-SB203580 only, which were evaluated at 8 hrs time point. At each time point, mRNA and protein expressions of RPAs of MCP-1 were measured. The phosphorylation of p38 MAPK (p-pMAPK) of RPAs was also detected.
Results: We found that the RPAs-TNF-α elicited significant increases in MCP-1 expression and phosphorylation of p38 mitogen-activated protein kinase (p-p38 MAPK). Furthermore, the MCP-1 expressions of RPAs-Resveratrol, RPAs-C1142, and RPAs-SB203580 were significantly down-regulated, which was associated with robustly suppressed TNF-α-induced p-p38MAPK expression.
Conclusion: Our findings suggested that MCP-1 was involved in the formation of TNF-α-induced inflammatory response, and resveratrol could down-regulate the expression of MCP-1 via TNF-α- inhibition, which might contribute to the decline of acute PTE-induced PH in vivo.
Keywords: inflammation, pulmonary artery endothelial cells, pulmonary hypertension, pulmonary thromboembolism, resveratrol
Introduction
Acute pulmonary thromboembolism (PTE) has a high mortality rate. PTE-induced pulmonary hypertension (PH) and acute pulmonary vascular injury are the main causes of high morbidity and mortality.1 Traditionally, the mechanical extent of pulmonary vascular occlusion caused by embolic thrombosis is considered to be a major determinant of the increase.1 However, recent studies have shown that the degree of pulmonary occlusion is inconsistent with PH. In particular, when the mechanical obstruction of pulmonary embolism is detached, PH seems to persist.2 Recent studies have found that the response of cytokines produced by infiltrating inflammatory cells to PTE increases pulmonary endothelial dysfunction, which plays an important role in the formation of PH.3 Monocyte chemoattractant protein-1 (MCP-1) is a key mediator stimulating the infiltration of inflammatory cells into the lung. In the early stages of PTE, MCP-1 levels are significantly augmented around the pulmonary arterial walls.4,5 Therefore, MCP-1 has been shown to be an important inflammatory regulator of vascular and pulmonary inflammation and may be closely related to the PH formation. The phytoalexin resveratrol (3,5,4ʹ-trans trihydroxystilbene) found in grapes mediates these anti-inflammatory effects.6 It suppresses MCP-1 synthesis and secretion in vascular endothelial cells7 and in an in vivo model of vascular inflammation.8 The p38 mitogen-activated protein kinase (p38MAPK) is involved in several processes that are thought to be important in the inflammatory response.9,10 Previous studies have demonstrated that p38MAPK is an important upstream signaling molecule of MCP-1, and inhibition of p38MAPK activation could down-regulate MCP-1 the expression.11,12 To solve this problem, we tested a p38MAPK-specific inhibitor SB203580 in this study. The purpose of this study was to investigate the effect of resveratrol on TNF-α induced-inflammatory response to provide insight into possible mechanisms whereby resveratrol could decrease TNF-α-induced inflammatory response by inhibiting p38MAPK activation and down-regulating MCP-1 expression in rat pulmonary artery endothelial cells (RPAECs).
Materials and methods
Reagents
A polyclonal rabbit anti-rat MCP-1 antibody of Abcam (http://www.abcam.com/), rabbit polyclonal antibodies for total and phospho-p38 MAPK [Cell Signaling Technology (http://www.cellsignal.com/)] and SB203580 and resveratrol of [Sigma–Aldrich (http://www.sigmaaldrich.com)] were purchased from the above companies. SYBR Green real-time PCR kit was from Toyobo (http://www.bio-toyobo.cn/). C1142 (a rodent chimeric antibody anti-rat MCP-1) was purchased from Centocor (http://www.centocororthobiotech.com). TNF-α was purchased from R&D Systems (http://www.rndsystems.com/).
Acute PTE model13
Male Sprague-Dawley adult rats (n=132) weighing 300–350 g, purchased from the laboratory animal center of Wenzhou Medical College, Wenzhou, Zhejiang, China, were used for the investigation. All the animal procedures were treated based on the guidelines of Wenzhou Medical College and National Institutes of Health standards of animal care. This protocol was approved by the Animal Ethics Committee of Wenzhou Medical College (Zhejiang SCXK, 2005–0,019,150).
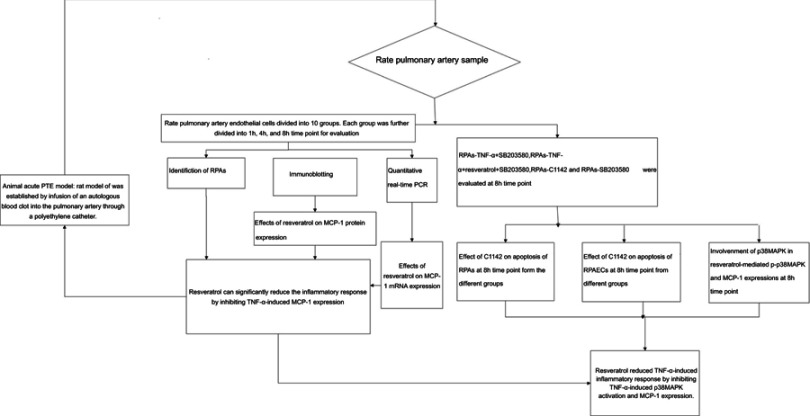
A rat model of acute PTE was established by infusion of an autologous blood clot into the pulmonary artery through a polyethylene catheter. Specific pathogen-free rats (n=132) were randomly and equally divided into ten groups: rats-control group (Untreated), rats-1% DMSO, rats-TNF-α, rats-TNF-α + resveratrol, rats-TNF-α + C1142, rats-TNF-α + SB203580, rats-TNF-α + resveratrol + SB203580, rats-resveratrol only, rats-C1142 only and S rats-B203580 only. Resveratrol (10 mg/kg/day, i.p.),14 C1142 (2 mg/kg/day, i.p.)15 or SB203580 (2.5 mg/kg/day, i.p.;)16 were administered to the animals for 1 hr prior to the start of the acute PTE protocol. In the rats-TNF-α + resveratrol + SB203580, we pre-treated rats with SB203580 and resveratrol for 1 hr prior to the start of the acute PTE protocol and then for another 8 hrs. Each group was divided into 1, 4, and 8 hrs time point for rat pulmonary artery endothelial cells (RPAs) evaluation (RPAs collected from six rats per time point) except for rats-TNF-α + SB203580, rats-TNF-α + resveratrol + SB203580, rats-C1142 only and rats-SB203580 only, which were evaluated 8 hrs time point only (n=6 per group) (Figure 1).
Figure 1.
Diagram of the study design showing the TNF-α-induced inflammatory response was reduced by resveratrol via down-regulation of the protein expression of MCP-1.
Rat pulmonary artery sample
RPAs were isolated from above animal and designated as: RPAs-control, RPAs were collected from six rats of the rats-control group at 1, 4, and 8 hrs point time, respectively; RPAs-1% DMSO control, RPAs were collected from six rats of the rats-1% DMSO, at 1, 4, and 8 hrs point time, respectively; RPAs-TNF-α, RPAs were collected from six rats of the rats-TNF-α at 1, 4, and 8 h point time, respectively; RPAs-TNF-α + resveratrol (Sigma–Aldrich, USA), RPAs were collected from six rats of the rats-TNF-α + resveratrol at 1, 4, and 8 hrs point time, respectively; RPAs-TNF-α + C1142, RPAs were collected from six rats of the rats-TNF-α + C1142 at 1, 4, and 8 hrs point time, respectively; RPAs-TNF-α + SB203580, RPAs were collected from six rats of the rats-TNF-α + SB203580 at 8 hrs point time; RPAs-TNF-α + resveratrol + SB203580, RPAs were collected from six rats of the rats-TNF-α + resveratrol + SB203580 at 8 hrs point time; RPAs-Resveratrol only, RPAs were collected from six rats of the rats-resveratrol only at 1, 4, and 8 hrs point time, respectively; RPAs-C1142 only, RPAs were collected from six rats of the rats-C1142 only at 8 hrs point time and RPAs-SB203580 only, RPAs were collected from six rats of the rats-B203580 only at 8 hrs point time. All operations were performed under sterile conditions (Figure 1).17
Cell identification by immunocytochemistry17
Immunocytochemistry for the subcultured RPAs (fourth generation, 40–50% confluence) on fibronectin-coated coverslips was performed according to the procedure described previously. Briefly, after PBS washing, the cells were fixed with 4% paraformaldehyde and then permeabilized with 0.25% Triton X-100. Following 1% BSA blocking for 30 mins at RT, the cells were incubated with 200 μL of primary antibodies of anti-von Willebrand factor rabbit polyclonal antibody (1:200) for 90 mins at RT. The cells were incubated with the appropriate HRP-conjugated secondary antibody [donkey anti-rabbit IgG (1:100)] for 30 mins at RT after three washes in PBS. Antigen-antibody complexes were observed with 3,3′ -diaminobenzidine (DAB) tetrahydrochloride after counterstained with hematoxylin for nuclei using rat PASMCs as positive controls for α-smooth muscle actin.
Terminal dUTP nick-end labeling (TUNEL) assay
PRAs slides were stained with the TUNEL technique using an in situ apoptosis detection kit (Cat#G7130; Promega, Madison, WI, USA) according to the manufacturer’s protocols with modifications to detect testicular DNA integrity. The slides were hydrated through graded ethanol (100%, 100%, 95%, 85%, 70%, and 50%) for 3 mins, followed by a wash for 5 mins with 0.85% NaCl and PBS, respectively. They were incubated with proteinase K (20 g/mL) for 15 min at room temperature. Thereafter, adding rTdT reaction mix onto the slides at 37°C for 1 hr in a humidified chamber to end labeling reaction. The slides immersed into the stop buffer for 15 mins at room temperature to terminate rTdT enzyme reaction, washed in PBS for 3×5 mins. Endogenous peroxidase was then blocked by 0.3% H2O2 PBS solution for 30 mins. After three times PBS washes, they were incubated with streptavidin-HRP solution in a humidified chamber for 30 mins at room temperature. Excess unbound streptavidin-HRP was removed by PBS washes for 3×5 mins. Chromogenic substrate 3,3ʹ-DAB solution was added onto the slides. The color development was monitored under an optical microscope, and the reaction was stopped by rinsing the slides in deionized water until the light brown background. All slides were dehydrated through graded series of ethanol solutions (50%, 70%, 80%, 95%, 100%, and 100%) for 3 mins each, cleared in 100% xylene for 3×5 mins each, and mounted in Neutral Balsam (Sinopharm Chemical Reagent Co., Ltd.). Staining was observed under a light microscope and use OLYMPUS DP71 digital camera and DP controller software to take all digital images.
Immunoblotting
After determination of total tissue protein concentration by BCA protein assay kit, 40 μg of the protein prepared above was separated on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, then transferred the separated protein was to a PVDF membrane, and using 5% fat free milk-TBST buffer blocked the membrane for 2 hrs at room temperature; incubated it with the rabbit antibodies of anti-MCP-1 (ab9669,1:500, Abcam, UK), anti-total p38MAPK (#8690, 1:1000, Cell Signaling Technology, USA), anti-p-p38MAPK (9211S,1:1000, Cell Signaling Technology, USA) and anti β-actin antibody (A2228, 1: 2000, Sigma–Aldrich, USA) at 4°C overnight, followed with 1:5,000 secondary goat anti-rabbit antibodies incubation for 2 hrs (ab6721, Abcam, UK). The phosphorylated p38MAPK/total p38MAPK ratio was designated as the in vitro increased in p-p38 MAPK activity. After color development, the image was analyzed using ChemiScope mini chemiluminescence meter to calculate the optical density of the target and the internal control band for the protein expression. Protein expression = integral optical density value of the target protein/integral optical density value of β-actin.
Quantitative real-time PCR
Quick-frozen rat pulmonary artery samples (100 mg) were extracted with Trizol (Gibco Life Technologies, USA) for total RNA. Reverse transcription was carried out using a Bio-Rad PCR system S1000 (Bio-Rad, USA), in which 1 μg of total RNA was reacted in a volume of 10 μL, including 2 μL of 5×RT buffer, 0.5 μL of RT enzyme mixture, and 0.5 μL of a primer mixture. The RNase-free water adjusted the final reverse transcription product to 10 μL. The forward primer of MCP-1 (Invitrogen Life Technologies, USA) was 5ʹ-GTCTCTGTCACGCTTCTG-3ʹ and the reverse was 5ʹ-TGCTGGTGATTCTCTTGTAG-3ʹ. The forward primer for GAPDH was 5ʹ-AGAACATCATCCCTGCATCC-3ʹ and the reverse was 5ʹ-TGGATACATTGGGGGTAGGA-3ʹ. Real-time PCR was carried out in the 7,500 Real-Time PCR instrument (Applied Biosystems, USA) including 1 cycle of initial denaturation at 95°C for 2 mins, 40 cycles of amplification with denaturation at 95°C for 15 s, annealing and extension at 62°C for 1 min. All the reactions were performed in triplicate and normalized with GAPDH. Fluorescence quantitative was determined by the Applied Biosystems 7500 System SDS Software using the standard curve method. 2−ΔΔCT method was used to calculate MCP-1 (CCL2) mRNA expression.
Statistical analyses
All data were presented as mean ± SD. One-way ANOVA followed by LSD-t was used for statistical analyses by SPSS version 16.0.1. p<0.05 indicated statistically significant.
Results
Identification of RPAECs
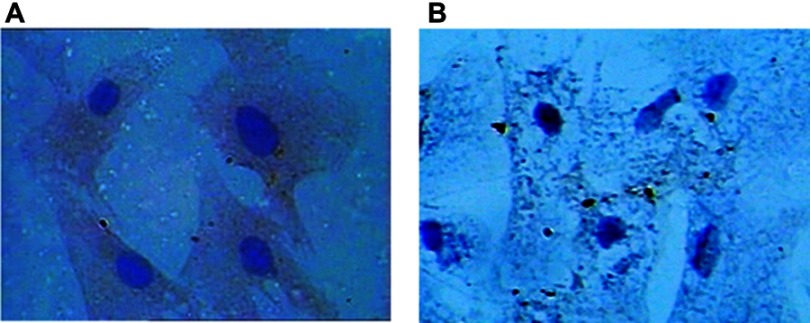
RPAs are center oval nuclei blue in color, polygonal, and spindle shaped. For anti-von Willebrand factor Cell immunity factor VIII related antibody staining, staining, positive cells showed brown cytoplasma (Figure 2).
Figure 2.
(A) Immunocytochemistry image of RPAs is polygonal and spindle-shaped cells presenting typical “paving-stone” and “pebbles” growth with blue ovoid nuclei in the center, anti-von Willebrand cellular immune staining showing that cytoplasm is tan positive (400×). (B) Negative control.
Abbreviation: RPAs, rat pulmonary artery endothelial cells.
Effects of resveratrol on MCP-1 protein expression
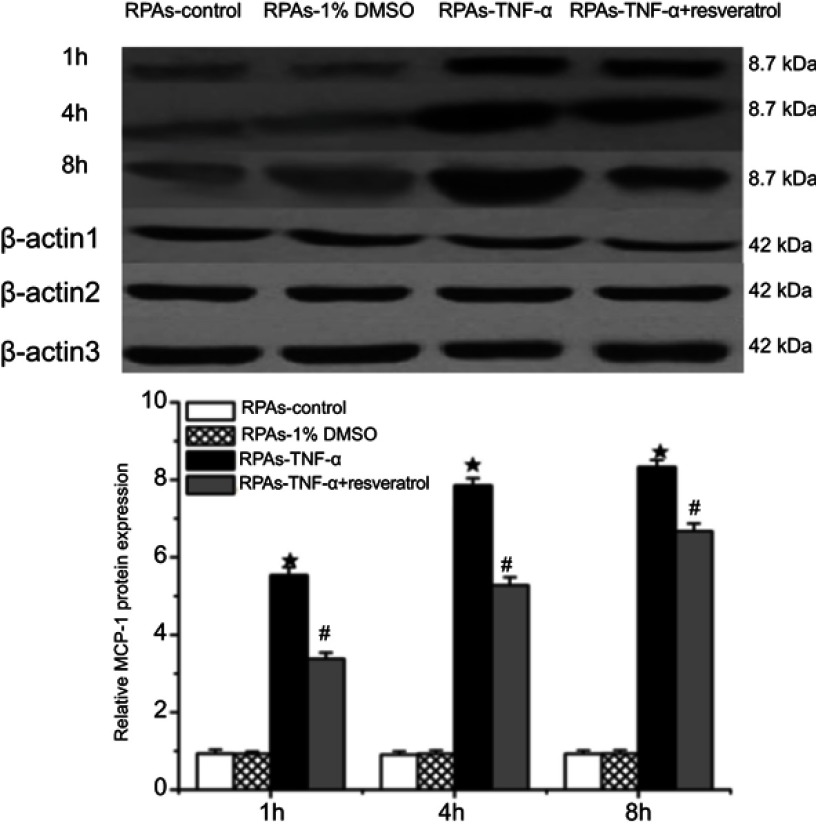
PARs Protein expression of MCP-1 was measured using immunoblotting. No difference in MCP-1 protein expression was observed between RPAs-control and RPAs-1% DMSO control. MCP-1 significantly increased in relation to the time of action in RPAs-TNF-α (★p<0.05 compared to the RPAs-control and RPAs-1% DMSO control). Remarkably, treatment with resveratrol down-regulated the protein expression of MCP-1, which was significantly lower than that RPAs-TNF-α (#p<0.05, RPAs-TNF-α + resveratrol compared to RPAs-TNF-α). RPAs-control and RPAs-1% DMSO control showed very low protein expression of MCP-1. The MCP-1 was mainly expressed in RPAs-TNF-α. In contrast, the MCP-1 expression in RPAs-TNF-α + resveratrol was down-regulated (Figure 3).
Figure 3.
Effects of resveratrol on MCP-1 protein expression. The MCP-1 protein levels in Pulmonary artery arteries were measured by immunoblotting at 1, 4, and 8 hrs time point. Β-actin was used to normalize protein loading (mean ± SD, n=6 rats each). ★p<0.05: RPAs-TNF-α vs RPAs-control and RPAs-1% DMSO; #p<0.05: RPAs-TNF-α vs RPAs-TNF-α + resveratrol.
Abbreviation: RPAs, rat pulmonary artery endothelial cells.
Effects of resveratrol on MCP-1 mRNA expression
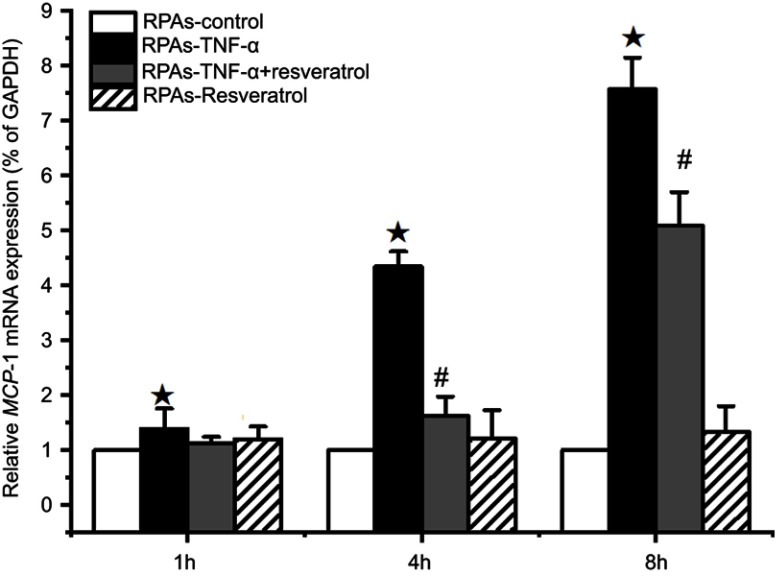
The relative amounts of MCP-1 mRNA expression in the RPAs were quantified by real-time PCR assay (Figure 4). MCP-1 mRNA expression in RPAs-TNF-α was significantly increased with prolongation of incubation time (P<0.05). On the contrary, treatment of resveratrol had a strong negative effect on the MCP-1 mRNA expression during the 4 and 8 hrs of the experiment (#p<0.05 RPAs-TNF-α + resveratrol compared with the RPAs-TNF-α and RPAs-Resveratrol Figure 3).
Figure 4.
Effect of resveratrol on MCP-1 mRNA expression of in pulmonary artery cells. The RPAs were treated with resveratrol, and MCP-1 mRNA expression was measured using real-time PCR assay at 1, 4, and 8 hrs time point (mean ± SD, n=6 each group). ★p<0.05: RPAs-TNF-α vs RPAs-control, RPAs-TNF-α + resveratrol, and RPAs-Resveratrol only; #p<0.05: RPAs-TNF-α + resveratrol vs RPAs-control and RPAs-Resveratrol only.
Abbreviation: RPAs, rat pulmonary artery endothelial cells.
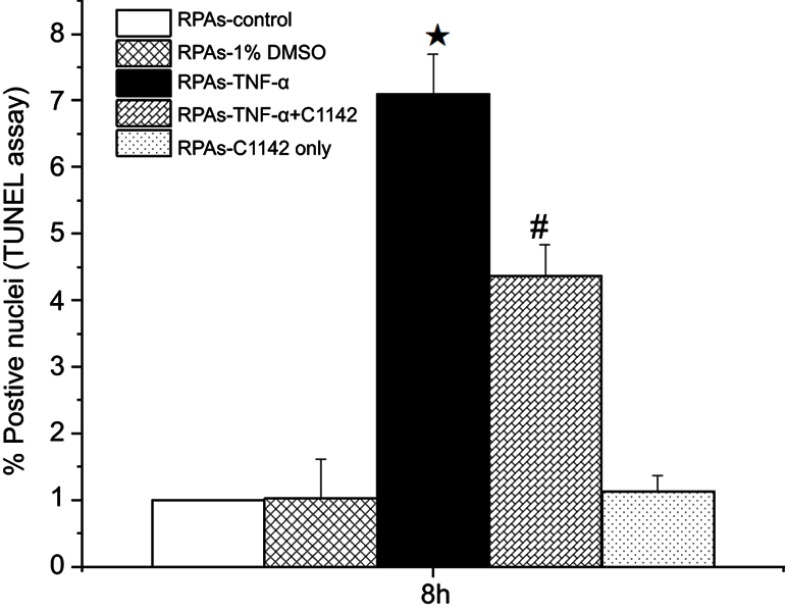
Effect of C1142 on apoptosis of RPAs at 8 hrs time point from different groups
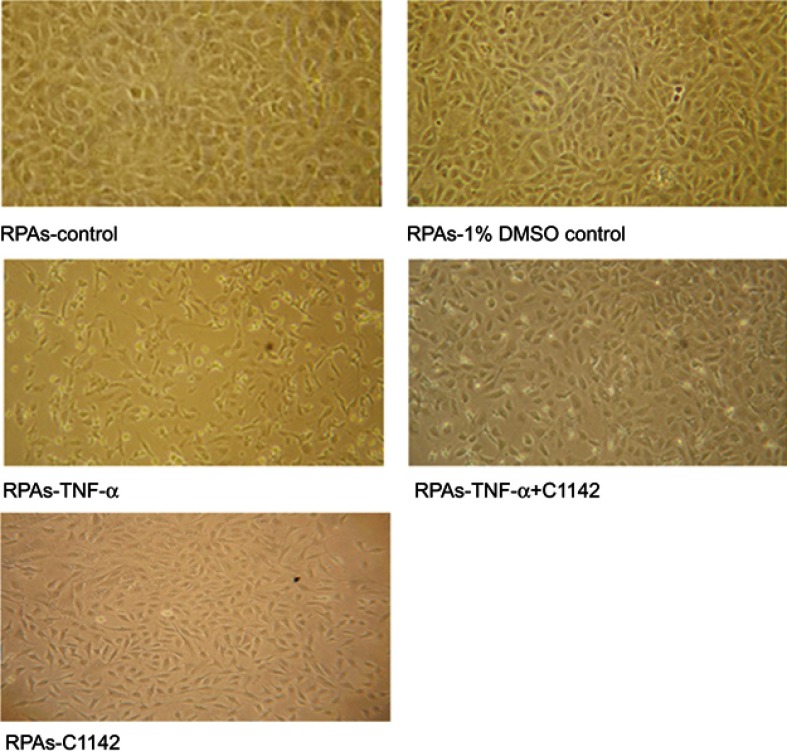
TUNEL assay showed that there was no significantly increased apoptosis of RPAs in at 8 hrs time point. The apoptosis of RPAs in RPAs-TNF-α and RPAs-TNF-α + C1142 were significantly increased compared to RPAs-control, RPAs-1% DMSO control, RPAs-TNF-α + C1142, and RPAs-C1142 only, P<0.05). Furthermore, C1142 (RPAs-TNF-α + C1142) significantly lowered apoptosis of RPAs at 8 hrs time point compared to RPAs-TNF-α; P<0.05, Figures 5 and 6; Table 1).
Figure 5.
The morphological images of RPAs at 8 hrs time point from different treated cells under the light microscope (100X) showed Effect of C1142 on apoptosis of RPAs at 8 hrs time point.
Abbreviation: RPAs, rat pulmonary artery endothelial cells.
Figure 6.
Effects of C1142 on apoptosis of RPAs at 8 hrs time point. ★/# p<0.05: RPAs-TNF-α and RPAs-TNF-α + C1142 vs RPAs-control, RPAs-1% DMSO control and RPAs-C1142 only; #p<0.05: RPAs-TNF-α + C1142 vs RPAs-TNF-α.
Abbreviation: RPAs, rat pulmonary artery endothelial cells.
Table 1.
The apoptosis index in RPAs from different groups (mean ± SD, n=6)
| Detection time | RPAs-control | RPAs-1% DMSO control | RPAs-TNF-α | RPAs-TNF-α + C1142 |
|---|---|---|---|---|
| 8 hrs | 4.31±0.35 | 4.36±0.37 | 5.09±0.41★ | 4.02±0.47# |
Notes: ★p<0.05: RPAs-TNF-α vs RPAs-1% DMSO control 2; #p<0.05: 4. RPAs-TNF-α + resveratrol vs RPAs-TNF-α.
Abbreviation: RPAs, rat pulmonary artery endothelial cells.
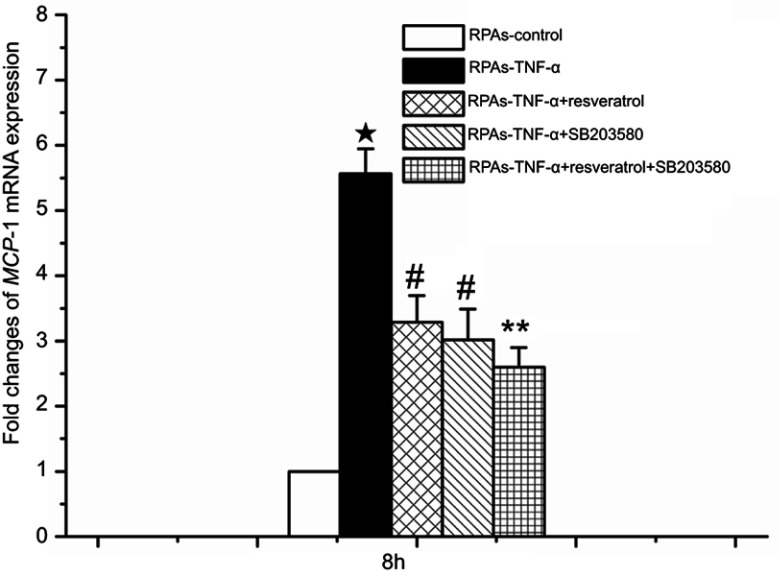
Involvement of p38MAPK in resveratrol-mediated p-p38MAPK and MCP-1 expressions at 8 hrs time point
The use of p38MAPK-specific inhibitor SB203580 was designed to confirm p38MAPK-mediated effects. This anti-inflammatory drug inhibits the catalytic activity of p38MAPK by competing for binding in the ATP pocket.18 It inhibits the catalytic activity of p38MAPK by competing for binding in the ATP pocket18 p-p38MAPK and MCP-1 expression levels at 8 hrs time point was investigated by immunoblotting and real time-PCR, because p-p38 MAPK and MCP-1 expression peaked at the time point of 8 hrs.
Figures 7 and 8 indicate that no significant differences were noted in p-p38MAPK and MCP-1 levels between the RPAs-control and the RPAs-1% DMSO control; however, it demonstrated a significant increase in p-p38MAPK and MCP-1 of RPAs-TNF-α (protein and mRNA expressions) compared to RPAs-control, RPAs-1% DMSO, RPAs-TNF-α + resveratrol, RPAs-TNF-α + SB203580, RPAs-Resveratrol, RPAs-SB203580, and RPAs-TNF-α + resveratrol + SB203580. But resveratrol and SB203580 significantly lowered their levels (#p<0.05 RPAs-TNF-α + resveratrol and RPAs-TNF-α + SB203580 compared to RPAs-TNF-α). In order to study the effect of p-p38MAPK on resveratrol-mediated MCP-1 mRNA and protein expressions, rats were pre-treated with SB203580 for 1 hr before TNF-α challenge. We noted that the expressions of p-p38MAPK and MCP-1 in the RPAs harvested from the SB203580 pretreated rat (RPAs-TNF-α + resveratrol + SB203580) were significantly decreased (#p<0.05 compared with RPAs-TNF-α). Co-treatment of SB203580 with resveratrol significantly diminished TNF-α induced p-p38MAPK and MCP-1 expressions. For both p-p38MAPK and MCP-1 expressions, there was statistical significance between the RPAs-TNF-α + resveratrol + SB203580 and RPAs-TNF-α + resveratrol and RPAs-TNF-α + SB203580 (P<0.05). These results suggest that p38MAPK signaling mediates MCP-1 expression. Consequently, resveratrol might decrease TNF-α-induced inflammatory response via inhibiting TNF-α-induced p38MAPK activation and MCP-1 expression. There were statistically significant differences between the RPAs-TNF-α + resveratrol and RPAs-TNF-α + SB203580 and the RPAs-TNF-α + resveratrol + SB203580 for p-p38MAPK and MCP-1 and MCP-1 expressions (**p<0.05, Figures 7 and 8). There were no significant changes observed in RPAs apoptosis, p38MAPK, p-p38MAPK, and MCP-1 when comparing the RPAs-Resveratrol only, RPAs-C1142 only and RPAs-SB203580 only with the RPAs-control.
Figure 7.
Effect of resveratrol on protein expression of MCP-1 and p-p38MAPK in pulmonary artery cells. Pulmonary artery arterial cells were obtained from rats treated with resveratrol, and levels of MCP-1protein expression were measured by immunoblotting at 8 hrs time point. β-actin was used to normalize protein loading (mean ± SD, n=6 each group). ★p<0.05: RPAs-TNF-α vs RPAs-control, RPAs-1% DMSO, RPAs-TNF-α + resveratrol, RPAs-TNF-α + SB203580, RPAs-Resveratrol, RPAs-SB203580, and RPAs-TNF-α + resveratrol + SB203580. #p<0.05: RPAs-TNF-α + resveratrol and RPAs-TNF-α + SB203580 vs RPAs-TNF-α; ** p<0.05: RPAs-TNF-α + resveratrol + SB203580 vs RPAs-TNF-α + resveratrol and RPAs-TNF-α + SB203580.
Abbreviation: RPAs, rat pulmonary artery endothelial cells.
Figure 8.
MCP-1 mRNA expression mediated by the p38 MAPK-dependent signaling pathway in pulmonary artery cells. The levels of MCP-1 mRNA expression were measured using real-time PCR assay at 8 hrs time point in pulmonary artery cells (mean ± SD, n=6 each group). ★p<0.05: RPAs-TNF-α vs RPAs-control, RPAs-TNF-α + resveratrol, RPAs-TNF-α + SB203580 and RPAs-TNF-α + resveratrol + SB203580groups. #p<0.05: RPAs-TNF-α + resveratrol and RPAs-TNF-α + SB203580 vs RPAs-control and RPAs-TNF-α + resveratrol + SB203580; **p<0.05: RPAs-TNF-α + resveratrol + SB203580 vs RPAs-control.
Abbreviation: RPAs, rat pulmonary artery endothelial cells.
Discussion
Acute PTE is due to embolization that blocks the pulmonary vasculature, causing acute pulmonary vascular injury and the resulting PH leading to large morbidity and mortality which created a substantial health care burden.1 Recent study indicated that the degree of pulmonary arterial obstruction is not consistent with the increase in pulmonary artery pressure.2 Even if the mechanical obstruction of the pulmonary embolism was relieved, PH still existed.19 Evidence of infiltration of inflammatory cells in response to PTE suggested that white blood cells may play an important role in the formation of PH.2 During PTE manifestation, thrombi trapped in pulmonary vessels would damage the vascular endothelium, thus causing the unregulated release of proinflammatory mediators.20 TNF-α is mainly produced by activated macrophages, which is an important marker for systemic inflammation. Blood level of TNF-α also remained significantly higher in patients with PE.21
In early acute PTE, there is a large amount of inflammatory cell infiltration accompanied by up-regulation of various types of cytokines and chemokines. The enhanced inflammation can promote the release of a series of inflammatory mediators, which in turn exacerbate pulmonary artery endothelium injury.11 Member of the chemokines C–C or beta subfamily MCP-1 induces monocyte recruitment, promotes cytokine production and forms an inflammatory positive feedback loop in acute pulmonary vascular injury.3,22 This may lead to the occurrence of PH. However, the ability of the lung to remove thromboembolism is considerable. By enzymatic activity such as fibrinolysis, the fibrin networks within the clot are dissolved and, particularly small but also larger emboli can be completely treated.23
In our previous research, we had demonstrated that when disengaged mechanical obstruction, PH lasts up to 8 hrs. At the same time, the expression of MCP-1 in the acute PTE group increased markedly. Based on these findings, we hypothesized that the MCP-1 and the formation of acute PTE-induced PH were closely correlated. Our previous results indicated that resveratrol likely decreased the acute PTE-induced PH via down-regulation of MCP-1 expression, reducing the recruitment of mononuclear cells, and attenuating pulmonary artery endothelial cell injury.16 Basal levels of MCP-1 were significantly decreased in the presence of resveratrol at 1–50 μM in a concentration-dependent manner and were significantly decreased in the presence of U0126, an ERK inhibitor.24
TNF-α has been demonstrated to be one of the main inflammatory mediators involved in autoimmune diseases, such as rheumatoid arthritis, systemic sclerosis. TNF-α initiates or aggravates inflammation by activating NF-κB and producing cytokines. Resveratrol inhibited the TNF-α induced acetylation of RelA/p65 via a Sirtuin1-dependent manner in 3T3 cells.25 We demonstrated the up-regulation of MCP-1 expression was participated in the formation of TNF-α induced inflammatory reaction in RPAs. Further, we found that administration of resveratrol could partially reduce inflammatory reaction, by inhibiting the acute TNF-α-induced p38MAPK activation and MCP-1 expression. These findings have for the significance in acute PTE-induced PH treatment. The data clearly shown that TNF-α could up-regulate MCP-1compared with the control group. Compared with the TNF-α group, our results clearly showed that C1142 could decrease the concentration of MCP-1. In vivo studies have shown that C1142 can reduce the mean pulmonary artery pressure, which indicates that in addition to vascular obstruction caused by thrombosis, the MCP-1 expression is related to the formation of acute PTE-induced PH. A therapeutic strategy that down-regulates MCP-1 expression in RPAs may aid in modulating the PH in response to acute PTE.
Resveratrol has a variety of pharmacological effects, including anti-inflammatory properties14 inhibition of foam cell formation via NADPH oxidase 1-mediated induction of MCP-1.26,27 and anti-inflammatory effect on TNF-α-induced MCP-1 expression. In this investigation, the inflammation model of acute PTE in vitro for the effect of resveratrol on the expression of MCP-1 was studied. Our results indicated that resveratrol may reduce the expression of MCP-1, the recruitment of monocytes and the injury of pulmonary artery endothelial cell. It is known that inhibition of p38MAPK activation decreases MCP-1 levels.10 Gresele et al, have found that the pro-inflammatory pathway associated with p38MAPK can be blunted by resveratrol in acute ischemic heart disease.28 It acts as an antioxidant by blocking oxidative stress-induced p38MAPK activation.29 To confirm that resveratrol inhibited TNF-α-induced p38MAPK activation and down-regulated MCP-1 expression, we pretreated rats with SB203580 and resveratrol. Co-treatment of SB203580 with resveratrol significantly reduced TNF-α-induced p-p38MAPK and MCP-1 expression. The expression of p-p38MAPK and MCP-1 was statistically significant between RPAs-TNF-α + SB203580+ resveratrol and RPAs-TNF-α + resveratrol, indicating that p38MAPK signaling mediates MCP-1 expression. Therefore, resveratrol reduced TNF-α-induced inflammatory response by inhibiting TNF-α-induced p38MAPK activation and MCP-1 expression.
Conclusion
In the formation of TNF-α-induced inflammatory response, MCP-1 was up-regulated. This study demonstrates for the first time that resveratrol can significantly reduce the inflammatory response by inhibiting TNF-α-induced MCP-1 expression in isolated pulmonary artery endothelial cells, which will help with the PH caused by the PTE reaction. Therefore, we speculate that resveratrol is likely to be used as a novel prophylactic anti-inflammatory agent for acute PTE-induced PH treatment.
Acknowledgments
This Project was supported by Zhejiang Natural Science Foundation (Q18H010007); Zhejiang Province Medical and Health Science and Technology Project (2018243886); and Zhejiang Education Department General Scientific Research Project (Y201738380).
Ethic Approval
The study was approved by the Animal Ethics Committee of Wenzhou Medical College (Zhejiang SCXK, 2005-0019 150).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res. 2000;48:23–33. [DOI] [PubMed] [Google Scholar]
- 2.Kimura H, Okada O, Tanabe N, et al. Plasma monocyte chemoattractant protein-1 and pulmonary vascular resistance in chronic thromboembolic pulmonary artery hypertension. Am J Respir Crit Care Med. 2001;164:319–324. doi: 10.1164/ajrccm.164.2.2006154 [DOI] [PubMed] [Google Scholar]
- 3.Langer F, Schramm R, Bauer M, Tscholl D, Kunihara T, Schäfers HJ. Cytokine response to pulmonary thromboendarterectomy. Chest. 2004;126:135–141. doi: 10.1378/chest.126.1.135 [DOI] [PubMed] [Google Scholar]
- 4.Eagleton MJ, Henke PK, Luke CE, et al. Inflammation and intimal hyperplasia associated with experimental pulmonary embolism. J Vasc Surg. 2002;36:581–588. [DOI] [PubMed] [Google Scholar]
- 5.Rectenwald JE, Deatrick KB, Sukheepod P, et al. Experimental pulmonary embolism: effects of the thrombus and attenuation of pulmonary artery injury by low-molecular-weight heparin. J Vasc Surg. 2006;43:800–808. doi: 10.1016/j.jvs.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 6.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 2007;275:218–220. doi: 10.1126/science.275.5297.218 [DOI] [PubMed] [Google Scholar]
- 7.Cullen JP, Morrow D, Jin Y, et al. Resveratrol, apolyphenolic phytostilbene, inhibits endothelial monocyte chemotacticprotein-1 synthesis and secretion. J Vasc Res. 2007;44:75–84. doi: 10.1159/000098155 [DOI] [PubMed] [Google Scholar]
- 8.Rius C, Abu-Taha M, Hermenegildo C, et al. Trans- but not cis-resveratrol impairs angiotensin-ii-mediated vascular inflammation through inhibition of nf-kappa b activation and peroxisome proliferator activated receptor-gamma upregulation. J Immunol. 2010;185:3718–3727. doi: 10.4049/jimmunol.1001043 [DOI] [PubMed] [Google Scholar]
- 9.Ulrich S, Loitsch SM, Rau O, et al. Peroxisome proliferator-activated receptor gamma as a molecular target of resveratrol-induced modulation of polyamine metabolism. Cancer Res. 2006;66:7348–7354. doi: 10.1158/0008-5472.CAN-05-2777 [DOI] [PubMed] [Google Scholar]
- 10.Green SR, Han KH, Chen Y, et al. The cc chemokine mcp-1 stimulates surface expression of cx3cr1 and enhances the adhesion of monocytes to fractalkine/cx3cl1 via p38 mapk. J Immunol. 2006;176:7412–7420. [DOI] [PubMed] [Google Scholar]
- 11.Shimada H, Rajagopalan LE. Rho-kinase mediates lysophosphatidic acid-induced il-8 and mcp-1 production via p38 and jnk pathways in human endothelial cells. FEBS Lett. 2010;584:2827–2832. doi: 10.1016/j.febslet.2010.04.064 [DOI] [PubMed] [Google Scholar]
- 12.Shui H, Gao P, Si XY, Ding GH. Mycophenolic acid inhibits albumin-induced mcp-1 expression in renal tubular epithelial cells through the p38 mapk pathway. Mol Biol Rep. 2010;37:1749–1754. doi: 10.1007/s11033-009-9599-y [DOI] [PubMed] [Google Scholar]
- 13.Runyon MS, Gellar MA, Sanapareddy N, Kline JA, Watts JA. Development and comparison of a minimally-invasive model of autologous clot pulmonary embolism in Sprague–dawley and Copenhagen rats. Thromb J. 2010;8:3. doi: 10.1186/1477-9560-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sener G, Topaloglu N, Sehirli AO, Ercan F, Gedik N. Resveratrol alleviates bleomycininduced lung injury in rats. Pulm Pharmacol Ther. 2007;20:642–649. doi: 10.1016/j.pupt.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 15.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. Ccl2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grumbles RM, Casella GTB, Rudinsky MJ, Godfrey S, Wood PM, Thomas CK. The immunophilin ligand fk506, but not the p38 kinase inhibitor sb203580, improvesfunction of adult rat muscle reinnervated from transplants of embryonic neurons. Neuroscience. 2005;130:619–630. doi: 10.1016/j.neuroscience.2004.09.024 [DOI] [PubMed] [Google Scholar]
- 17.Chun C, Yang W, Xueding C, et al. Resveratrol downregulates acute pulmonary thromboembolism-induced pulmonary artery hypertension via p38 mitogen-activated protein kinase and monocyte chemoattractant protein-1 signaling in rats. Life Sciences. 2012;90:721–727. doi: 10.1016/j.lfs.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 18.Lee JC, Kumar S, Griswold DE, et al. Inhibition of p38 map kinase as a therapeutic strategy. Immunopharmacology. 2000;47:185–201. [DOI] [PubMed] [Google Scholar]
- 19.Watts JA, Zagorski J, Gellar MA, Stevinson BG, Kline JA. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol. 2006;41:296–307. doi: 10.1016/j.yjmcc.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 20.Battistini B. Modulation and roles of the endothelins in the pathophysiology of pulmonary embolism. Can J Physiol Pharmacol. 2003;81:555–569. doi: 10.1139/y03-017 [DOI] [PubMed] [Google Scholar]
- 21.Halici B, US Sarinc, Gunay E, et al. Assessment of inflammatory biomarkers and oxidative stress in pulmonary thromboembolism: follow-up results. Inflammation. 2014;37:1186–1190. doi: 10.1007/s10753-014-9871-8 [DOI] [PubMed] [Google Scholar]
- 22.Strieter RM, Kunkel SL, Keane MP, Standiford TJ. Chemokines in lung injury Thomas A. Neff lecture. Chest. 1999;116:103–110. doi: 10.1378/chest.116.suppl_1.103S [DOI] [PubMed] [Google Scholar]
- 23.Wagenvoort CA. Pathology of pulmonary thromboembolism. Chest. 2005;107:10–17. doi: 10.1378/chest.107.1_Supplement.10S [DOI] [PubMed] [Google Scholar]
- 24.Wakabayashi I, Takeda Y. Inhibitory effects of resveratrol on MCP-1, IL-6, and IL-8 production in human coronary artery smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 2013;386(9):835–839. doi: 10.1007/s00210-013-0877-9 [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Liu Q, Wang M, et al. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS One. 2011;6(11):e27081. doi: 10.1371/journal.pone.0027081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park DW, Baek K, Kim JR, et al. Resveratrol inhibits foam cell formation via nadph oxidase 1-mediated reactive oxygen species and monocyte chemotactic protein-1. Exp Mol Med. 2009;41:171–179. doi: 10.3858/emm.2009.41.3.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J, Yong W, Wu XH, et al. Anti-inflammatory effect of resveratrol on tnf-alpha-induced mcp-1 expression in adipocytes. Biochem Biophys Res Commun. 2008;369:471–477. doi: 10.1016/j.bbrc.2008.02.034 [DOI] [PubMed] [Google Scholar]
- 28.Gresele P, Pignatelli P, Guglielmini G, et al. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J Nutr. 2008;138:1602–1608. doi: 10.1093/jn/138.9.1602 [DOI] [PubMed] [Google Scholar]
- 29.Chakraborty PK, Mustafi SB, Raha S. Pro-survival effects of repetitive low-grade oxidative stress are inhibited by simultaneous exposure to resveratrol. Pharmacol Res. 2008;58:281–289. doi: 10.1016/j.phrs.2008.08.007 [DOI] [PubMed] [Google Scholar]