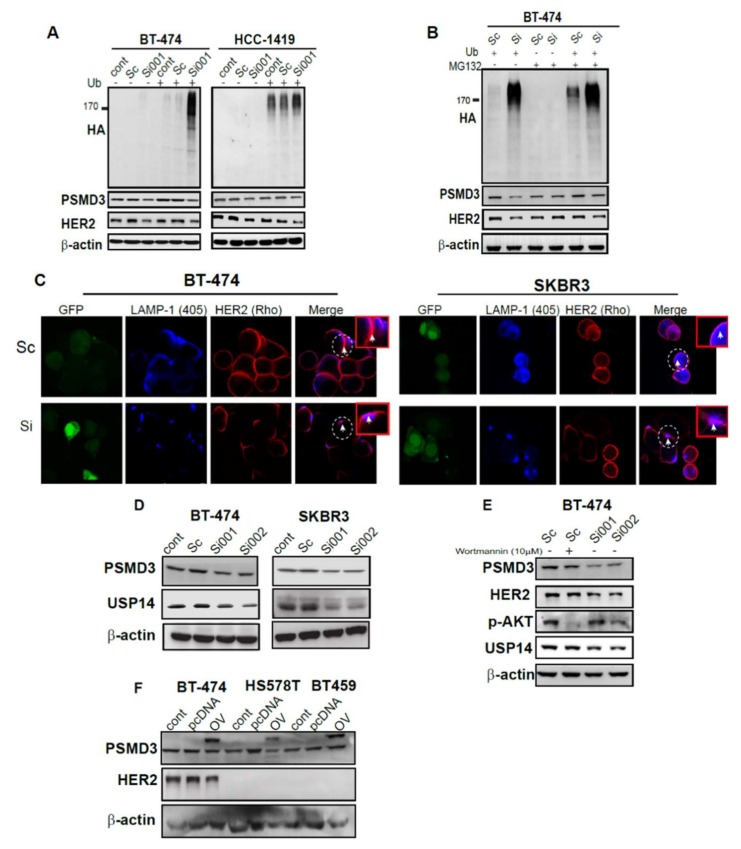
Figure 5.
Enhancing the ubiquitination and lysosomal degradation pathway through silencing of PSMD3. (A) BT-474 and HCC-1419 control. Scramble and PSMD3-Si001 were co-transfected with or without ubiquitin plasmid for 24 h and then lysed. The cell lysates were incubated with antibodies against Ubiquitin (HA), HER2, and PSMD3. β-actin served as a control. (B) BT-474 was treated with Scramble and PSMD3-Si001 were co-transfected with or without ubiquitin plasmid for 24 h and then treated with or without MG132 at a concentration of 5µM for 8 hours and lysed. The cell lysate was incubated with the indicated antibodies. (C) Confocal microscope pictures of SKBR3 and BT474 treated with scramble or PSMD3 for 24 h and incubated with HER2 and LAMP-1 lysosomal marker antibodies and showing HER2-LAMP-1 co-localization. Scale bar is 25 µM. (D) B-474, SKBR3 cell lysates were incubated with PSMD3 and USP14 antibodies. Western blot analysis to detect PSMD3 and USP14 protein levels was used. β-actin served as a control. (E) B-474 transfected with Scramble, PSMD3 si001, or PSMD3 si002 for 24 h. Control cells were treated with Wortmanine inhibitor for 3 hours. Western blot analysis to detect PSMD3, HER2, p-AKT, and USP14 protein levels was used. β-actin served as a control. (F) Overexpression of PSMD3 in BT474, HS578T, and BT459. Western blot data showing the expression of PSMD3, HER2, and β-actin; * indicates the PSMD3 conjugated with Yellow fluorescent protein (YFP) fusion protein, where the band shifted upward.