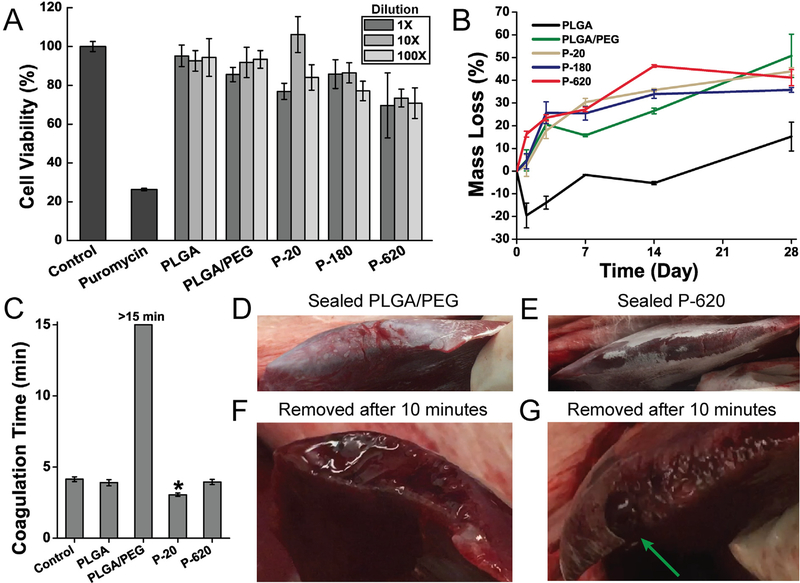
Fig. 6.
Polymer blend surgical sealants incorporating silica particles (denoted “P-X”, where “X” is the diameter of the silica particles incorporated into PLGA/PEG) have acceptable cell viability, appropriate degradation rate, and enhance hemostasis in vitro and in vivo. (A) Cell viability of L929 fibroblasts exposed to simulated in vitro 24 h extractions from various polymer-particle composite sealants. (B) Mass loss from fiber mats incubated at 37 °C in phosphate buffered saline (PBS). (C) Coagulation time measured by time to form a mechanically stable clot in an inverted vial. Asterisk indicates statistically significant difference between indicated group and all other groups. (D–G) In vivo comparison of hemostatic efficacy in a porcine liver laceration model. Immediately after resection, the liver surface was sprayed with polymer blend surgical sealant without silica particles (PLGA/PEG, D) and with 620 nm silica particles (P-620, E). (F) When PLGA/PEG was removed after 10 min, the resected area of the liver had not achieved hemostasis. (G) P-620 caused coagulation across the surface of the resection except for at a large hepatic vein, which is indicated by the green arrow. Composite surgical sealants are denoted “P-X”, where “X” is the diameter of the silica particles incorporated into PLGA/PEG. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)