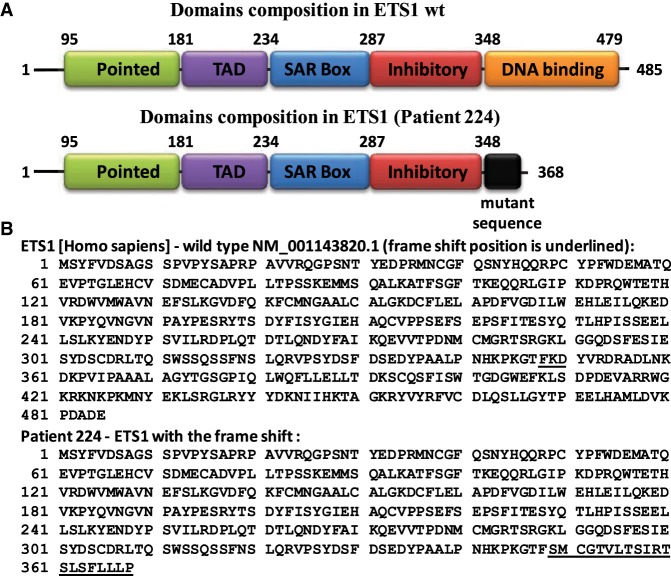
Figure 1.
ETS1 wild-type protein sequence and the same protein sequence after frameshift (Pt). (A) Structure of human ETS1 protein with previously defined domains. The pointed domain is involved in binding of ERK kinases to ETS1; the TAD domain is responsible for transactivation; the SAR domain is proposed to interact with SAR-interacting protein (SIP); the inhibitory domain suppresses ETS1 binding to DNA; and the DNA-binding domain (or ETS domain) is responsible for recognition and binding to the specific DNA sequences. (B) Amino acid sequence of wild-type human ETS1 protein and amino acid sequence of c.1044_1049delCAAGGAinsTT variant (patient) with additional mutant sequence (20-amino acids long—underlined).