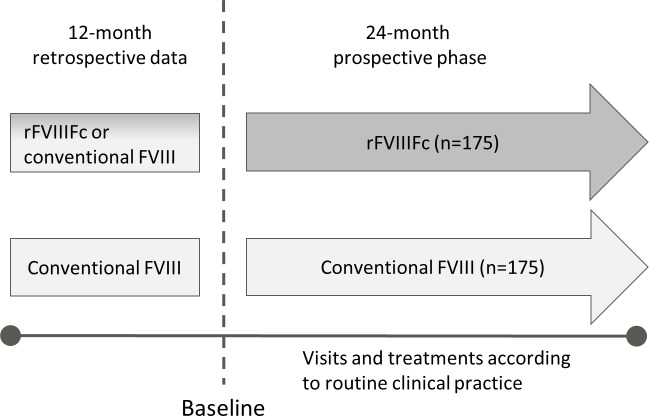
Figure 1.
A-SURE study design. A-SURE is a 24-month prospective observational study also including the collection of 12 months prebaseline data. The prophylactic factor treatment will be prescribed according to usual clinical practice and patients prescribed recombinant factor VIII Fc (rFVIIIFc) will be enrolled in the rFVIIIFc treatment arm and matched with patients with similar baseline characteristics receiving conventional coagulation factor VIII (FVIII) therapy.