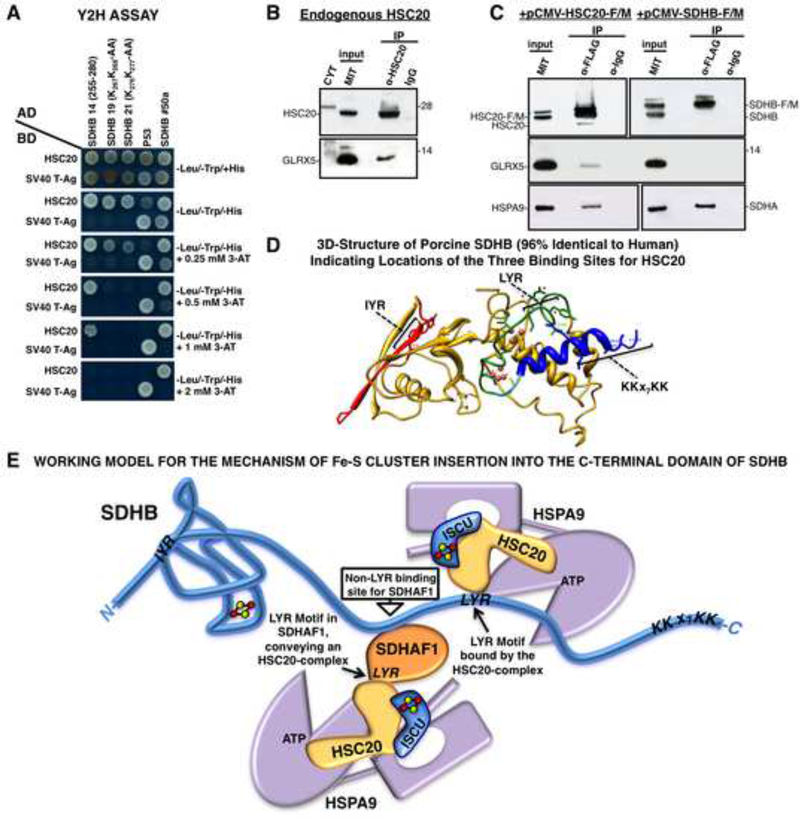
Figure 7. Residues 255–280 in the C-domain of SDHB Contain a Third Binding Site for HSC20.
(A) Y2H on clone 14 of SDHB (255–280), and on peptides in which KK residues of the KKx7KK motif were mutagenized. Mutagenesis of either the first or second KK dipeptide impaired binding to HSC20 (clones 19 and 21). (B, C) Endogenous or overexpressed HSC20 interacts with GLRX5 in vivo (A-C, n = 5 biological samples). (D) 3D-structure of porcine SDHB (96% identical to human SDHB. PBD ID: 3SFD) showing spatial distribution of HSC20 binding motifs. (E) Working model for the possible mechanism of Fe-S cluster insertion into the C-terminal domain of SDHB. See also Figure S6 and Table S3.