Figure 4. Potential workflow for clinical application of different ctDNA assays.
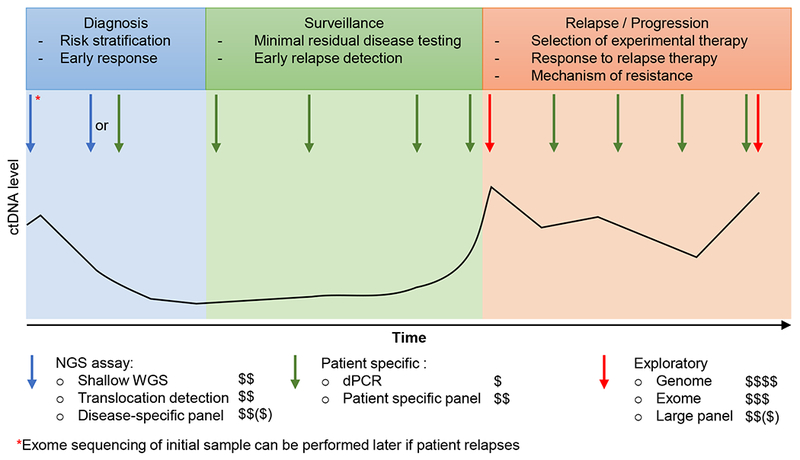
This graph depicts one potential strategy to combine complementary technologies to detect, quantify, and profile ctDNA throughout the course of a patient’s care. At diagnosis and early in therapy, focused NGS assays can be used to detect and quantify ctDNA without requiring existing genomic data from the tumor (blue arrows). Highly-sensitive patient-specific assays can be developed for use later in therapy to detect minimal residual disease and for surveillance (green arrows). Broad genomic profiling can performed on ctDNA at relapse or progression (red arrows) and compared to broad profiling of the initial diagnostic sample (red asterisk) to identify patterns of tumor evolution, treatment resistance, and identify new targetable variants for clinical trial enrollment. Dollar signs indicate relative cost of each approach. The black line indicates ctDNA levels in the patient throughout the course of treatment.
