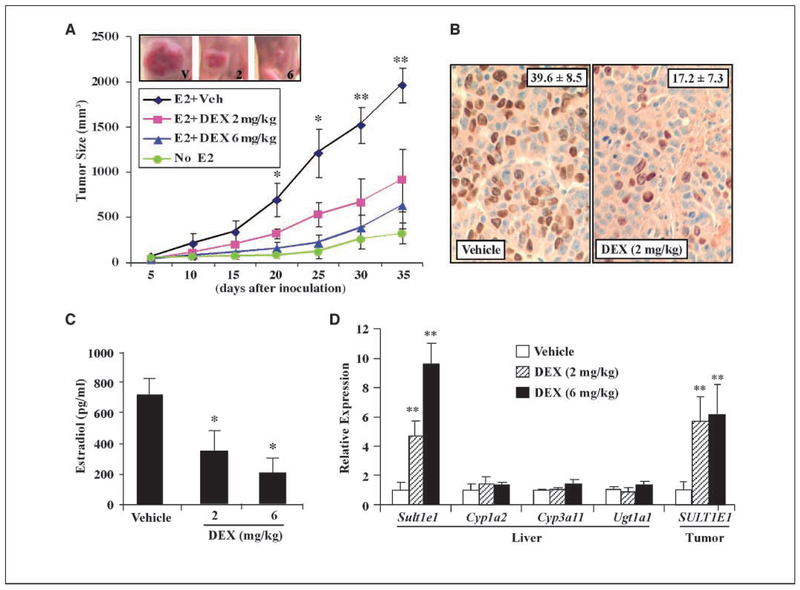
Figure 4.
DEX inhibited estrogen-dependent human breast cancer cell tumorigenicity in nude mice. A, growth kinetics of the MCF-7/VEGF tumors in the presence of indicated hormone and drug treatments. The DEX treatment groups received daily treatment of DEX at indicated doses by i.p. injection. Tumor volumes were measured at the indicated times. Results are presented as means ± SD. Each group contains at least nine mice. *, P < 0.05; **, P < 0.01, compared with the DEX treatment group. Shown in the insert are representative E2-induced tumors in vehicle-treated or DEX-treated mice with the DEX doses labeled. B, BrdUrd immunostaining in E2-induced tumors 25 d postinoculation. Quantitation of BrdUrd-positive nuclei is labeled. Three mice of each group from A were selected for BrdUrd immunostaining. C, serum levels of E2 from mice in A at the time of sacrifice (35 d postinoculation). *, P < 0.05. At least five mice of each group were used forE2 measurement. D, DEX treatment increased hepatic and MCF-7/VEGF tumor expression of Sult1e1/SULT1E1, but not for Cyp1a2, Cyp3a11, and Ugt1a1.**, P < 0.01. Three mice of each group were used for gene expression analysis.