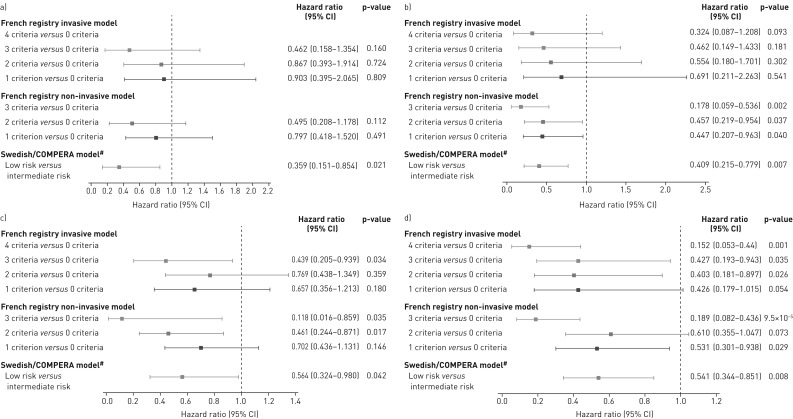
FIGURE 2.
Forest plots comparing survival in PATENT-2 expressed as hazard ratios (95% CI) for mortality comparing patients who achieved one or more low-risk criteria compared with those who achieved no low-risk criteria at a) baseline and b) follow-up in PATENT-1, and clinical worsening-free survival in PATENT-2 expressed as hazard ratios (95% CI) for clinical worsening comparing patients who achieved one or more low-risk criteria compared with those who achieved no low-risk criteria at c) baseline and d) follow-up in PATENT-1. Only patients receiving riociguat 2.5 mg three times daily maximum in PATENT-1 who participated in PATENT-2 were considered in this analysis. French registry invasive method: number of low-risk criteria fulfilled: 6-min walk distance (6MWD) >440 m, World Health Organization (WHO) functional class (FC) I/II, right atrial pressure (RAP) <8 mmHg and cardiac index ≥2.5 L·min−1·m−2. French registry non-invasive method: number of low-risk criteria fulfilled: 6MWD >440 m, WHO FC I/II and N-terminal pro-brain natriuretic peptide (NT-proBNP) <300 pg·mL−1. Swedish/COMPERA method: mean of grades (1–3: low, intermediate, high) of six available criteria (6MWD, WHO FC, NT-proBNP, RAP, cardiac index and mixed venous oxygen saturation) as defined in the European Society of Cardiology/European Respiratory Society 2015 treatment guidelines, rounded to the nearest integer. #: due to the small number of patients in the high-risk category at baseline and follow-up according to the Swedish/COMPERA method, these patients were not included in this analysis. Due to no or few events in some risk groups/strata, hazards ratios could not be calculated in some cases.