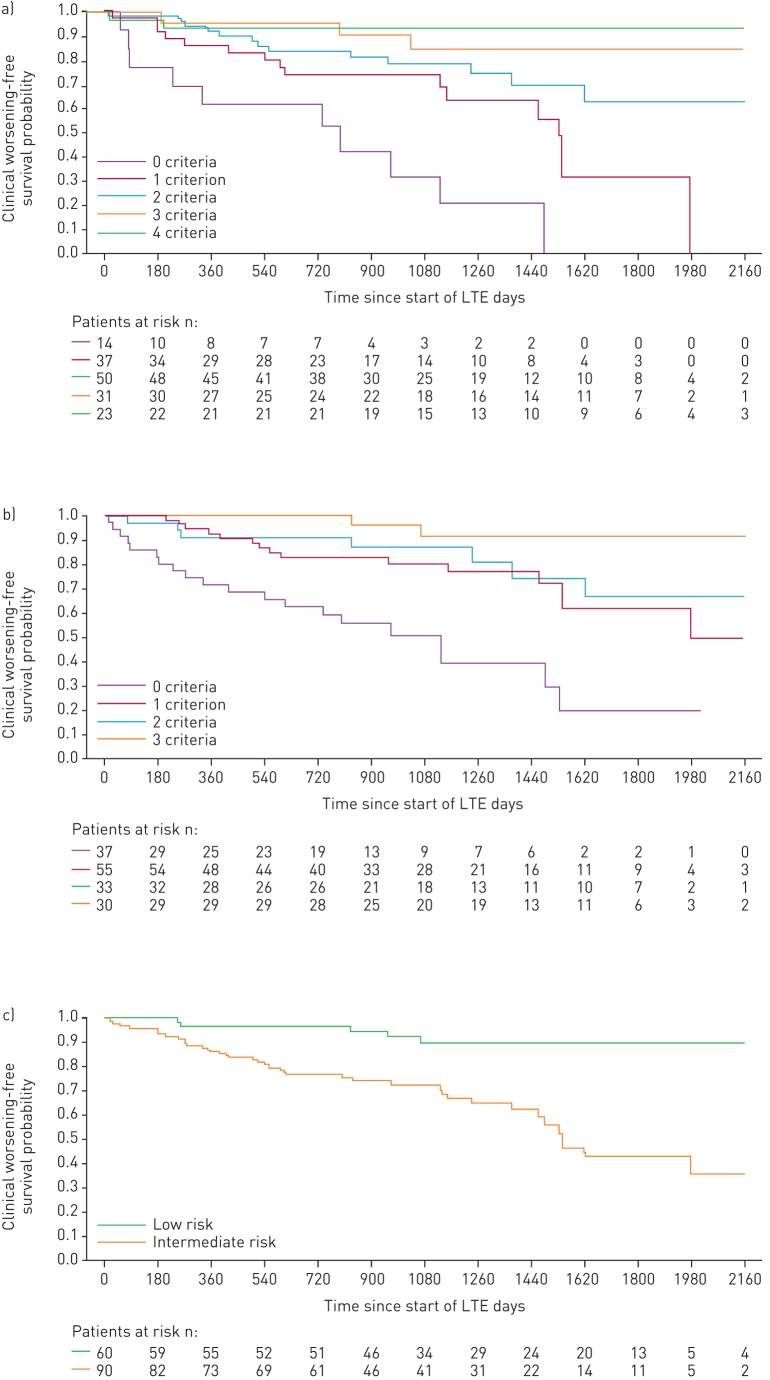
FIGURE 6.
Kaplan–Meier curves for clinical worsening-free survival in patients based on risk stratification at CHEST-1 follow-up: a) French registry invasive method, b) French registry non-invasive method and c) Swedish/COMPERA method. Only patients receiving riociguat 2.5 mg three times daily maximum in CHEST-1 who participated in CHEST-2 were considered in this analysis. Data were based on observed cases with no imputation. Day 0=start of extension study. Only five patients were in the Swedish/COMPERA high-risk group at CHEST-1 follow-up and were therefore not included in the analysis for this method. French registry invasive method: number of low-risk criteria fulfilled: 6-min walk distance (6MWD) >440 m, World Health Organization (WHO) functional class (FC) I/II, right atrial pressure (RAP) <8 mmHg and cardiac index ≥2.5 L·min−1·m−2. French registry non-invasive method: number of low-risk criteria fulfilled: 6MWD >440 m, WHO FC I/II and N-terminal pro-brain natriuretic peptide (NT-proBNP) <300 pg·mL−1. Swedish/COMPERA method: mean of grades (1–3: low, intermediate, high) of six available criteria (6MWD, WHO FC, NT-proBNP, RAP, cardiac index and mixed venous oxygen saturation) as defined in the European Society of Cardiology/European Respiratory Society 2015 treatment guidelines, rounded to the nearest integer. Log-rank test: invasive p<0.0001, non-invasive p<0.0001 and Swedish/COMPERA p<0.0001. LTE: long-term extension.