Figure 5.
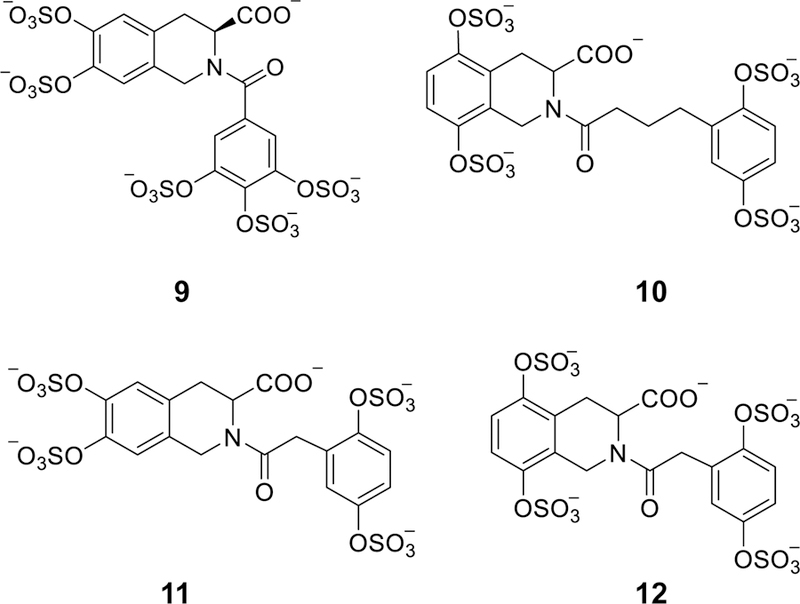
The chemical structures of the second generation of THIQ-based ATIII activators (9–12). Pharmacophore approach was exploited to design this generation. Structural variations were as follows: positions of the two sulfate groups on THIQ scaffold (5,6-, 6,7-, and 5,8-), the presence or absence of carboxylate group at position-3, the linker length between the bicyclic and monocyclic units (1-, 2-, 3-, and 4-atom linkers), and the number and position of sulfate groups on the monocyclic unit (2 or 3 sulfates) and (2,5-disulfate, 3,4-disulfate, and 3,4,5-trisulfate groups). Considering the acceleration potential, THIQ-N-acyl derivative 10 accelerated ATIII inhibition of FXa by ~80-fold, only 3.75-fold less than that achieved by DEF unit under similar condition.
