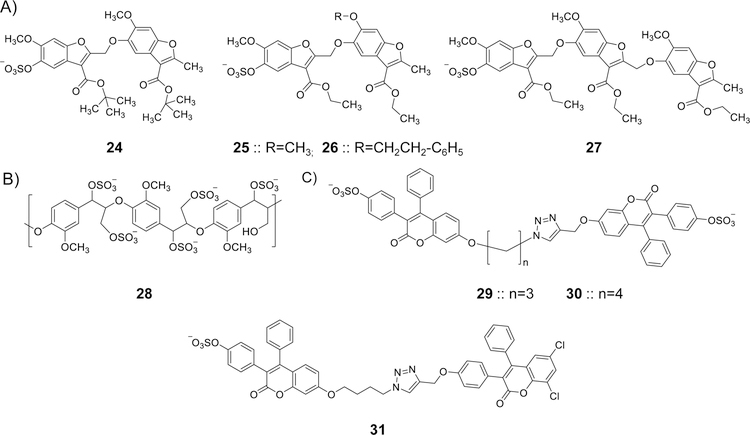
Figure 8.
The chemical structures of various allosteric inhibitors of thrombin. A) Monosulfated benzofuran dimers (24-26) and trimers (27) were designed considering the structural and mechanistic aspects of LMWLs. Extensive biochemical work revealed that sulfated NSGMs bind to exosite II of thrombin in similar fashion to heparin, yet in contrast to heparin, they allosterically disrupt the active site’s catalytic triad. Trimer 27 is the most potent inhibitor in this class of sulfated NSGMs with IC50 value of 0.67 µM and efficacy of 79%. A 2-fold increase in APTT and PT required 139 and 568 μM of inhibitor 27, respectively. B) A sulfated β-O4 lignin (SbO4L) 28 was intuitively designed as a mimic of the sulfated tyrosine sequence of GPIbα. Extensive biochemical studies established that this polymeric sulfated NSGMs is an allosteric, exosite II-binding inhibitor of human thrombin and a competitive inhibitor of GPIbα-mediated platelet aggregation. SbO4L has presented unique anticoagulant and antiplatelet properties which were confirmed by studies in human plasma, human whole blood, FeCl3-induced carotid arterial thrombosis model in mice, and Rose Bengal–laser injury model of arterial thrombosis in mice. C) The chemical structures of a new generation of sulfated coumarin dimers (29–31) that partially and allosterically modulate the catalytic activity of human thrombin. This was established by extensive biochemical studies. Dimer 31 is the most potent inhibitor with an IC50 value of 0.2 µM (KD=143 nM) and efficacy of 47%.