Figure 9.
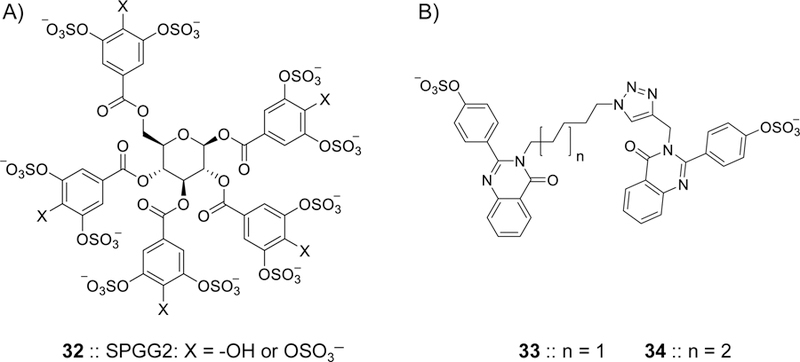
Chemical structures of allosteric inhibitors of FXIa. A) SPGG2 32 is a sulfated galloid-based NSGM. SPGG2 variant is the first small molecule allosteric inhibitor of FXIa. SPGG2 inhibited human FXIa with an IC50 value of 0.5 µM and a selectivity index of at least 200-fold against a range of coagulation, fibrinolysis, and digestive proteins. Extensive biochemical studies established the structural and mechanistic properties of this molecule. SPGG2 binds to the anion-binding site in the catalytic domain of FXIa. Its anticoagulant activity was confirmed in human normal and deficient plasmas as well as in human whole blood using thrombo-elastography technology. B) Sulfated quinazolinone dimers 33 and 34 were reported as allosteric inhibitors of FXIa. They were structurally designed using the dual-element recognition approach. Sulfated quinazolinone homodimers were identified to have micromolar potencies toward FXIa and significant selectivity against thrombin, FXa, chymotrypsin, and trypsin. In different studies, NSGM 32 inhibited the HSV-1 entry into multiple cell lines demonstrating antiviral activity that was comparable to acyclovir.
