Key Points
Question
Is a circulating biomarker of visceral fat associated with metabolic health changes to endurance exercise training?
Findings
This combination of cross-sectional and longitudinal analyses of an existing exercise study assessed the association between a novel circulating marker of hepatic fat, dimethylguanidino valeric acid, and metabolic health traits before and after 20 weeks of endurance exercise training. Dimethylguanidino valeric acid levels identified individuals with metabolic dysfunction at a young age and are associated with an adverse response in high-density lipoprotein traits and insulin sensitivity to exercise training.
Meaning
Blood dimethylguanidino valeric acid levels may identify individuals less responsive to the metabolic health benefits of endurance exercise training.
This cross-sectional analysis of an endurance exercise trial investigates the associations between dimethylguanidino valeric acid levels and metabolic health traits before and after 20 weeks of endurance exercise training.
Abstract
Importance
Metabolic responses to exercise training are variable. Metabolite profiling may aid in the clinical assessment of an individual’s responsiveness to exercise interventions.
Objective
To investigate the association between a novel circulating biomarker of hepatic fat, dimethylguanidino valeric acid (DMGV), and metabolic health traits before and after 20 weeks of endurance exercise training.
Design, Setting, and Participants
This study involved cross-sectional and longitudinal analyses of the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) Family Study, a 20-week, single-arm endurance exercise clinical trial performed in multiple centers between 1993 and 1997. White participants with sedentary lifestyles who were free of cardiometabolic disease were included. Metabolomic tests were performed using a liquid chromatography, tandem mass spectrometry method on plasma samples collected before and after exercise training in the HERITAGE study. Metabolomics and data analysis were performed from August 2017 to May 2018.
Exposures
Plasma DMGV levels.
Main Outcome and Measures
The association between DMGV levels and measures of body composition, plasma lipids, insulin, and glucose homeostasis before and after exercise training.
Results
Among the 439 participants included in analyses from HERITAGE, the mean (SD) age was 36 (15) years, 228 (51.9%) were female, and the median (interquartile range) body mass index was 25 (22-28). Baseline levels of DMGV were positively associated with body fat percentage, abdominal visceral fat, very low-density lipoprotein cholesterol, and triglycerides, and inversely associated with insulin sensitivity, low-density lipoprotein cholesterol, high-density lipoprotein size, and high-density lipoprotein cholesterol (range of β coefficients, 0.17-0.46 [SEs, 0.026-0.050]; all P < .001, after adjusting for age and sex). After adjusting for age, sex, and baseline traits, baseline DMGV levels were positively associated with changes in small high-density lipoprotein particles (β, 0.14 [95% CI, 0.05-0.23]) and inversely associated with changes in medium and total high-density lipoprotein particles (β, −0.15 [95% CI, −0.24 to −0.05] and −0.19 [95% CI, −0.28 to −0.10], respectively), apolipoprotein A1 (β, −0.14 [95% CI, −0.23 to −0.05]), and insulin sensitivity (β, −0.13; P = 3.0 × 10−3) after exercise training.
Conclusions and Relevance
Dimethylguanidino valeric acid is an early marker of cardiometabolic dysfunction that is associated with attenuated improvements in lipid traits and insulin sensitivity after exercise training. Levels of DMGV may identify individuals who require additional therapies beyond guideline-directed exercise to improve their metabolic health.
Introduction
Regular exercise is associated with improvements in metabolic health, including insulin sensitivity, lipid metabolism, and body composition.1,2,3 Despite the abundance of beneficial effects that exercise imparts on metabolism, substantial interindividual heterogeneity exists in the response to structured physical activity.4,5 Even standardized exercise training programs produce large differences in cardiorespiratory fitness and metabolic health responses, and data to guide exercise therapies toward targeted health outcomes are limited.5,6,7,8,9 The mechanistic underpinnings of regular exercise’s beneficial health outcomes remain poorly understood. Thus, illuminating the biochemical pathways involved in chronic exercise–induced adaptations remains an important goal of the medical community.10,11
By integrating human genetics and nontargeted metabolomics profiling, we recently identified a poorly characterized metabolite, dimethylguanidino valeric acid (DMGV), as a circulating biomarker of liver fat.12 Dimethylguanidino valeric acid is generated through the transamination of asymmetric dimethylarginine, which participates in nitric oxide signaling and vascular biology.13,14 We found that circulating levels of DMGV are higher in individuals with nonalcoholic steatohepatitis and are associated with incident type 2 diabetes up to 12 years prior to disease onset in 3 population-based cohorts.12 Furthermore, DMGV levels decreased significantly in participants after weight loss surgery, demonstrating its responsiveness to a beneficial metabolic intervention. These findings raise additional questions about the temporal association of DMGV with the onset of metabolic disease and whether more readily available lifestyle interventions such as exercise may be associated with its levels.
Thus, we sought to investigate DMGV’s association with metabolic health, including its response to exercise training in the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) Family Study. The HERITAGE study is a completed 20-week endurance exercise clinical trial performed in adult members of nuclear families who were sedentary and did not have cardiometabolic disease in which detailed measures of anthropometric measurements, body composition, lipid traits, and insulin and glucose homeostasis were assessed before and after exercise training. This study presents a unique opportunity to examine the association between DMGV and rich metabolic phenotypes in healthy parents and their biologic offspring at baseline and in response to regular endurance exercise. We specifically tested whether exercise training lowers DMGV levels in conjunction with improved metabolic health traits, and whether baseline DMGV levels could be used to assess prospective responses of metabolic traits to exercise training.
Methods
HERITAGE Family Study
Participants
The HERITAGE Family Study was a multicenter study designed to investigate the role of human variation in cardiometabolic responsiveness to regular exercise. Full details of its exclusion criteria, study design, and protocol have been described previously.15 Briefly, 99 white families that included both biologic parents (≤65 years old) and 2 or 3 offspring (≥17 years old) were tested, exercise-trained for 20 weeks, and retested. Participants were sedentary and free from cardiovascular and metabolic disease at study enrollment. In the current study, metabolomic assessments were performed in 441 white participants (166 parents and 273 offspring) who completed the exercise training intervention.
Written informed consent was obtained from all participants in the HERITAGE Family Study. The HERITAGE study consent form was reviewed and approved by Beth Israel Deaconess Medical Center's institutional review board. The same board reviewed the consent form from all the human study collaborators invovlved in this analysis to determine that the research performed was consistent with the scope of original consent.
Exercise Training Program
A detailed description of the exercise program has been provided elsewhere.16 Briefly, participants underwent supervised training on a cycle ergometer 3 days a week for 20 weeks (for 60 total sessions). Maximal oxygen uptake was directly measured during 2 cycle ergometer tests performed at baseline and after training, and a mean value was determined. Participants trained at progressively increasing intensity and duration, beginning with 30 minutes per session at a heart rate associated with 55% of maximal oxygen uptake and ending with 50 minutes per session at 75% of maximal oxygen uptake during the last 6 weeks of training.
Clinical Phenotyping
Anthropometric measurements and body composition, plasma lipids, lipoproteins, apolipoproteins, lipoprotein subclass and particle sizes, and measures of insulin and glucose homeostasis from an intravenous glucose tolerance test were assessed before and after exercise training. A detailed description of these measurements is provided in eMethods 1 in the Supplement. A complete list of the clinical traits examined is provided in eTable 1 in the Supplement.
Metabolomics Profiling of DMGV
Plasma DMGV levels were measured before and after training in 441 participants of the HERITAGE Family Study using a liquid chromatography, tandem mass spectrometry method previously used by our group.12 Details of the metabolomics methods are provided in eMethods 2 in the Supplement.
Statistics
Clinical characteristics are presented as frequencies and percentages for categorical data, means and SDs for normally distributed continuous variables, and medians (interquartile ranges [IQRs]) for nonnormally distributed continuous variables. Comparisons between continuous variables were performed with paired t tests or the Wilcoxon rank sum test as appropriate. Two-tailed P values less than .05 were considered significant. To approximate a normal distribution, DMGV levels were standardized to the nearest pooled plasma metabolite value within the cohort and natural logarithmically (log) transformed. Nonnormally distributed clinical measures were log transformed.
Linear regression was performed to determine the association between DMGV levels (the independent variable) and clinical traits (the dependent variables). Analyses included baseline DMGV levels and baseline clinical traits, baseline DMGV levels associated with changes (after exercise training) in clinical traits, and changes in DMGV levels correlated with changes in clinical traits. The DMGV levels were standardized to multiples of 1 SD. Regression models for baseline clinical traits included the covariates age, sex, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), abdominal visceral fat, and fasting insulin. For analyses with longitudinal changes in clinical traits, the baseline level of each trait and the changes in BMI were also examined. We used a statistical threshold of 1.1 × 10−3 (0.05/45 clinical traits) for significance in cross-sectional analyses and considered .05 nominally significant. For exploratory analyses with clinical trait changes, we used a statistical threshold of .05. Changes in DMGV after exercise testing were assessed by a paired t test. The association between baseline DMGV levels and changes in DMGV levels after exercise training was assessed using Pearson correlation analysis. All statistical analyses were performed using R version 3.3.1 (R Foundation for Statistical Computing). Metabolomics and data analysis were performed from August 2017 to May 2018.
Results
HERITAGE Cohort Characteristics
A total of 439 white participants who completed 20 weeks of exercise training were included in the analyses; 2 participants were excluded for having metabolite levels more than 8 SDs from the mean and more than 4 SDs from the next nearest value. The mean (SD) age of the entire cohort was 36 (15) years, and 228 were female (51.9%).
Clinical characteristics before and after training for parents, offspring, and the total cohort are shown in the Table. Significant improvements before and after exercise in maximal oxygen uptake (mean [SD], 33 [9] vs 39 [10] mL/kg/min; P < .001), body fat percentage (mean [SD]: 26.4% [10.2%] vs 25.8% [10.2%]; P < .001), abdominal visceral fat (median [IQR], 76 [45-121] cm2 vs 71 [43-111] cm2; P < .001), fasting insulin (median [IQR], 8.2 [6.1-11.2] μIU/mL vs 7.5 [5.5-10.5] μIU/mL; P < .001; to convert to picomoles per liter, multiply by 6.945), and apolipoprotein A1 (median [IQR], 118 [16] g/dL vs 122 [16] g/dL; P < .001; to convert to grams per liter, multiply by 0.01) were seen in the full cohort. Results of additional metabolic health responses to exercise training in the HERITAGE study have been published.6,7,8,17
Table. Health, Risk Factors, Exercise Training, and Genetics Study Clinical Characteristics Before and After Exercise Training.
| Clinical Characteristics | Whole Cohort (N = 439) | Parents (n = 166) | Offspring (n = 273) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | P Value | Before | After | P Value | Before | After | P Value | |
| Age, mean (SD), y | 36 (15) | NA | NA | 53 (5) | NA | NA | 26 (6) | NA | NA |
| Female, % | 228 (52) | NA | NA | 85 (51) | NA | NA | 143 (52) | NA | NA |
| Blood pressure, mean (SD), mm Hg | |||||||||
| Systolic | 116 (11) | 116 (11) | .44 | 119 (13) | 120 (13) | .18 | 115 (9) | 114 (9) | .83 |
| Diastolic | 66 (8) | 66 (8) | .16 | 70 (8) | 70 (9) | .92 | 63 (7) | 64 (7) | .11 |
| Peak oxygen uptake | |||||||||
| Mean (SD), ml/kg/min | 33 (9) | 39 (10) | <.001 | 27 (6) | 32 (7) | <.001 | 37 (8) | 43 (9) | <.001 |
| Median (IQR), L/min | 2.3 (1.9-3.0) | 2.8 (2.2-3.5) | <.001 | 2.3 (1.6-2.6) | 2.6 (1.9-3.0) | <.001 | 2.5 (2.0-3.2) | 3.0 (2.4-3.7) | <.001 |
| BMI, median (IQR) | 25 (22-28) | 25 (22-28) | .01 | 27 (25-31) | 27 (24-31) | .05 | 24 (21-27) | 23 (21-26) | .08 |
| Body fat, mean (SD), % | 26.4 (10.2) | 25.8 (10.2) | <.001 | 32.0 (8.6) | 31.5 (8.5) | <.001 | 27.3 (8.1) | 22.3 (9.6) | <.001 |
| Abdominal visceral fat, median (IQR), cm2 | 76 (45-121) | 71 (43-111) | <.001 | 126 (87-175) | 113 (82-164) | <.001 | 54 (35-82) | 51 (34-78) | <.001 |
| Fasting glucose, mean (SD), mg/dL | 91 (10) | 92 (10) | .24 | 95 (12) | 96 (12) | .78 | 88 (8) | 89 (8) | .19 |
| Fasting insulin, median (IQR), μIU/mL | 8.2 (6.1-11.2) | 7.5 (5.5-10.5) | <.001 | 8.5 (6.3-11.7) | 7.6 (5.3-10.9) | <.001 | 8.1 (5.9-10.8) | 7.5 (5.6-10.5) | <.001 |
| Insulin sensitivity, median (IQR) | 3.7 (2.3-5.9) | 4.1 (2.6-6.0) | <.001a | 3.2 (2.0-5.2) | 3.5 (2.3-5.4) | .05a | 4.0 (2.7-6.1) | 4.4 (2.8-6.3) | .005a |
| Total cholesterol, mean (SD), mg/dL | 174 (37) | 176 (36) | .03 | 194 (32) | 195 (32) | .50 | 162 (34) | 164 (33) | .02 |
| High-density lipoprotein cholesterol, mean (SD), mg/dL | 44 (10) | 44 (11) | .04 | 45 (11) | 45 (11) | .76 | 43 (10) | 44 (11) | .02 |
| Apolipoprotein A1, mean (SD), g/dL | 118 (16) | 122 (16) | <.001 | 122 (17) | 125 (16) | .001 | 116 (16) | 120 (16) | <.001 |
| Triglycerides, median (IQR), mg/dL | 106 (75-148) | 103 (74-144) | .33 | 124 (94-179) | 120 (90-171) | .03 | 91 (69-128) | 93 (68-127) | .75 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; NA, not applicable.
SI conversion factors: To convert glucose to millimoles per liter, multiply by 0.0555; insulin to picomoles per liter, multiply by 6.945; high-density lipoprotein cholesterol to millimoles per liter, multiply by 0.0259; apolipoprotein A1 to grams per liter, multiply by 0.01; and triglycerides to millimoles per liter, multiply by 0.0113.
Wilcoxon rank sum tests as appropriate; measurements before and after exercise training were compared using paired t tests.
Association of DMGV Levels With Cardiometabolic Risk Factors at Baseline
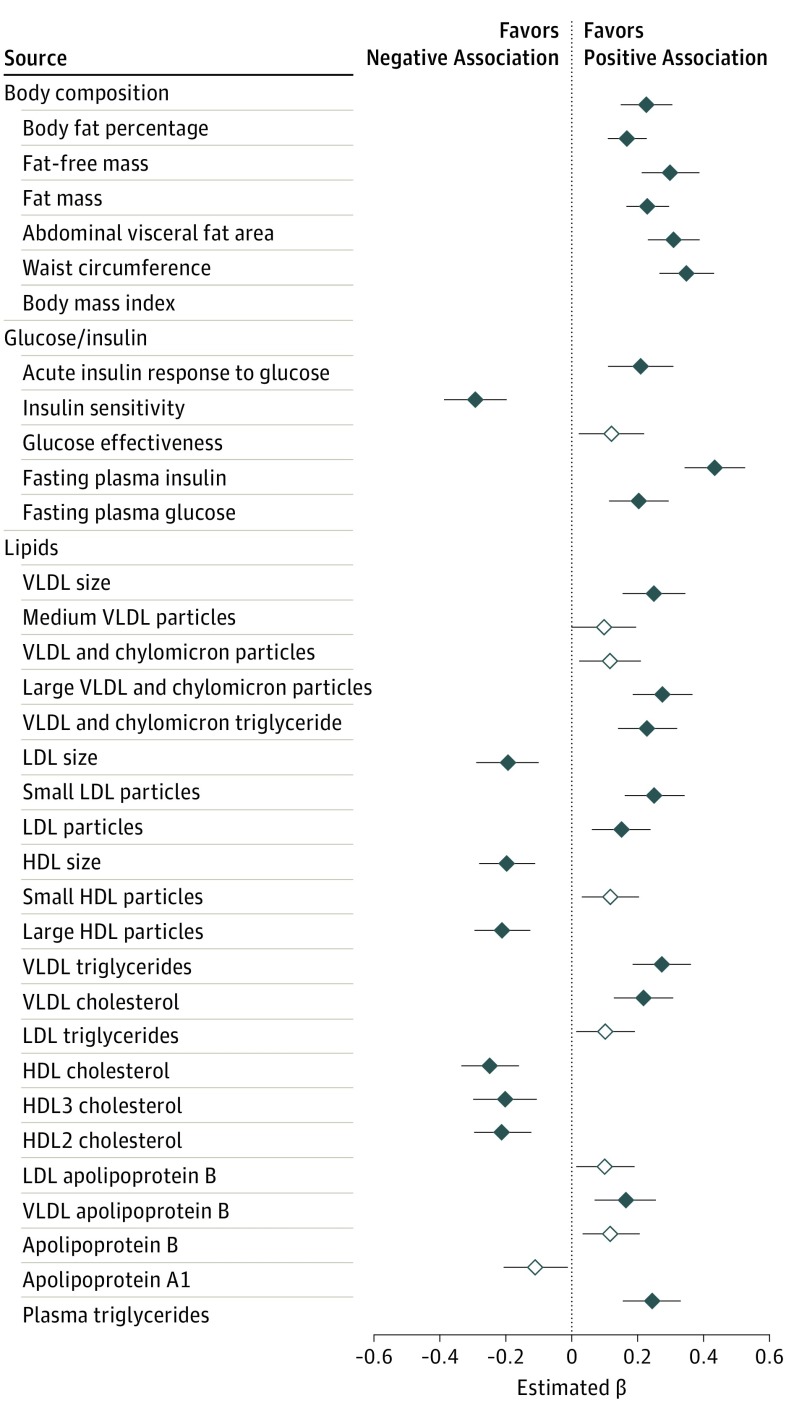
In age-adjusted, sex-adjusted analyses, baseline DMGV levels were positively correlated with adverse body weight and composition, lipid and lipoprotein, and glucose and insulin homeostasis traits (β coefficient ranges for associations meeting Bonferroni statistical significance: 0.17-0.46 [SE, 0.026-0.050]; nominal significance: 0.12-0.15 [SE, 0.042-0.052]; Figure 1). After adjustment for both BMI and abdominal visceral fat, correlations with all of the traits of glucose and insulin metabolism and 16 of the 22 traits of lipid metabolism remained highly significant (β coefficient [SE]: fasting insulin, 0.31 [0.048]; very low-density lipoprotein [VLDL] triglycerides, 0.23 [0.048]; large VLDL and chylomicron particles, 0.23 [0.05]; VLDL size, 0.23 [0.053]; VLDL and chylomicron triglycerides, 0.21 [0.05]; VLDL cholesterol, 0.18 [0.50]; high-density lipoprotein [HDL] 3 cholesterol, −0.19 [0.054]; HDL cholesterol, −0.17 [0.048]; low-density lipoprotein [LDL] size, −0.18 [0.053]; triglycerides, 0.17 [0.049]; small LDL particles, 0.16 [0.048]) or nominally significant (data in eFigure 1 in the Supplement). Similarly, additional adjustment for fasting insulin attenuated the associations with measures of insulin and glucose homeostasis; however, associations with lipid traits remained significant (β coefficient [SE] for VLDL size, 0.16 [0.054]; large VLDL and chylomicron particles, 0.16 [0.051]; VLDL and chylomicron triglycerides, 0.14 [0.051]; LDL size, −0.13 [0.054]; small LDL particles, 0.11 [0.049]; VLDL cholesterol, 0.11 [0.05]; HDL cholesterol, −0.1 [0.048]; HDL3 cholesterol, −0.11 [0.055]; eTable 2 in the Supplement). We further examined the association of DMGV with these traits in the offspring sample (mean [SD] age, 26 [6] years, median [IQR] BMI, 24 [21-27]) and found that DMGV remained positively associated with body fat percentage (β coefficient [SE], 0.31 [0.051]), abdominal visceral fat (0.27 [0.047]), triglycerides (0.21 [0.059]), and VLDL size (0.29 [0.059]) and was inversely associated with insulin sensitivity (−0.27 [0.061]), HDL cholesterol, (−0.16 [0.057]), HDL size (−0.19 [0.052]), and LDL size (−0.13 [0.06]; eFigure 2 in the Supplement).
Figure 1. Associations Between Dimethylguanidino Valeric Acid Levels and Baseline Metabolic Traits.
Effect sizes for each clinical trait are reported per SD increment of dimethylguanidino valeric acid levels in a linear regression model adjusted for age and sex. Black bars with solid diamonds meet Bonferroni statistical significance (P < .001); black bars with open diamonds meet nominal significance (P < .05). HDL indicates high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein.
DMGV Levels After Exercise Training and Correlations With Longitudinal Changes in Clinical Traits
We found that DMGV levels decreased after exercise training in both generations (eTable 3 in the Supplement). Baseline levels of DMGV were inversely correlated with their changes after exercise training (Pearson r = −0.42, P = 3.1 × 10−20; eFigure 3 in the Supplement). Decreases in DMGV levels were associated with decreases across a broad panel of lipid and glucose and insulin traits at the completion of the exercise program (eTable 4 in the Supplement). The changes in VLDL and chylomicron triglycerides (β [SE], 0.29 [0.043]), VLDL cholesterol (β [SE], 0.25 [0.045]), plasma triglycerides (β [SE], 0.24 [0.045]), medium and total HDL particles (HDL-P) (β [SE], 0.19 [0.043] and 0.18 [0.045]), apolipoprotein B (β [SE], 0.16 [0.044]), and LDL particles (β [SE], 0.11 [0.045]) remained positively associated with the changes in DMGV levels after exercise after further adjustment for baseline BMI and the changes in BMI after exercise training.
Association of DMGV Levels With Lipid and Insulin Sensitivity Improvements After Exercise Training
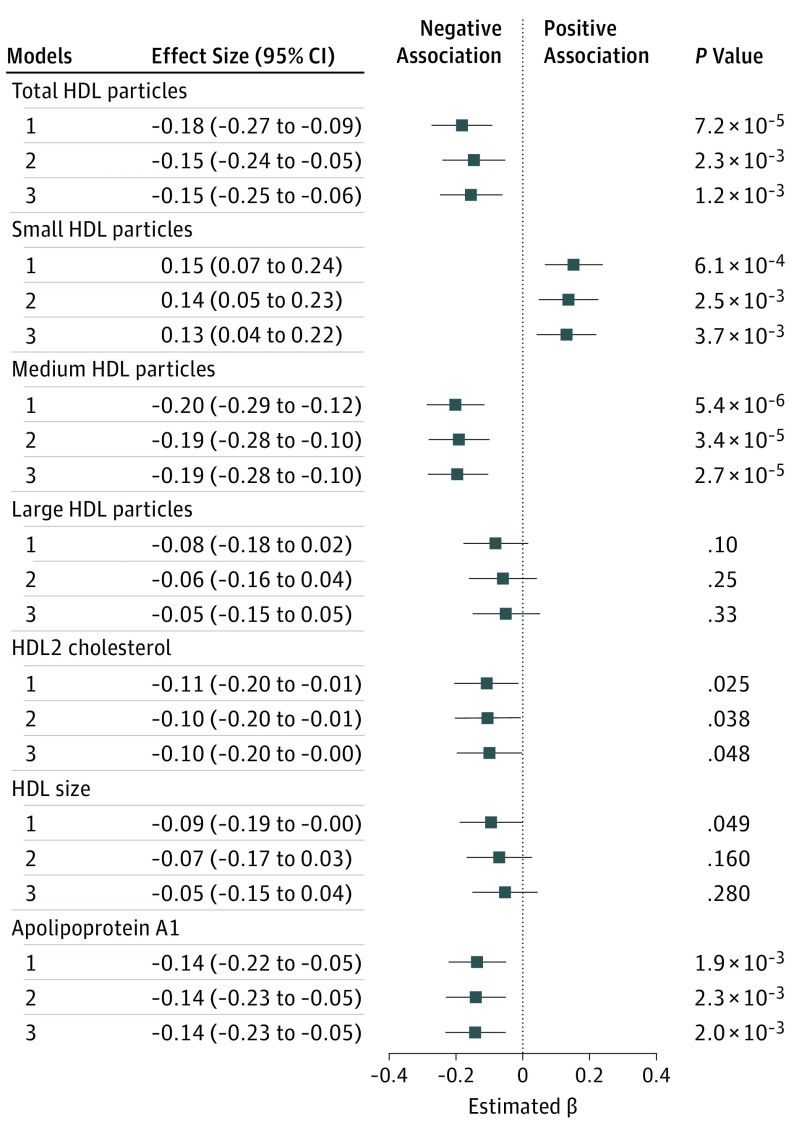
Baseline DMGV levels were inversely associated with the changes in total HDL-P (β, −0.15 [95% CI, −0.24 to −0.05]), medium HDL-P (−0.19 [95% CI, −0.28 to −0.10]), HDL2 (−0.10 [95% CI, −0.20 to −0.01]), and apolipoprotein A1 (−0.14 [95% CI, −0.23 to −0.05]) and positively associated with changes in small HDL-P (0.14 [95% CI, 0.05-0.23]) in a model adjusting for age, sex, and baseline level of each outcome variable (Figure 2). In a fully adjusted model that further included BMI and the changes in BMI after exercise, DMGV levels remained inversely associated with total HDL-P (β = −0.11; P = .03), medium HDL-P (β = −0.19; P = 2.7 × 10−5), and apolipoprotein A1 (β = −0.14; P = .002), and positively associated with small HDL-P changes (β = 0.13; P = .004). These associations were similar among parents and offspring. We tested whether the prognosticative capacity of DMGV for these traits was different than that of fasting insulin, given the strong correlation of DMGV with fasting insulin levels. We found that fasting insulin levels had a slightly weaker association to changes in medium HDL-P (insulin: β, −0.12; P = .01; DMGV, β, −0.19; P = 2.7 × 10−5) and were not associated with changes in the total HDL-P, apolipoprotein A1, or small HDL-P (eTable 5 in the Supplement). Given that HERITAGE participants had more prominent decreases in visceral fat than BMI with exercise training, we adjusted for baseline and changes in abdominal visceral fat in place of BMI in the fully adjusted model. The associations were unchanged (results not shown).
Figure 2. Associations Between Dimethylguanidino Valeric Acid Levels and High-Density Lipoprotein (HDL) Traits Responsiveness After 20 Weeks of Exercise Training in the Health, Risk Factors, Exercise Training, and Genetics Study Cohort.
Effect sizes for each clinical trait are reported per SD increment of dimethylguanidino valeric acid level based on a generalized linear model adjusted for age and sex. Model 1 is adjusted for baseline levels of each clinical trait. Model 2 further adjusts for age and sex. Model 3 further adjusts for baseline body mass index and changes in body mass index after exercise training.
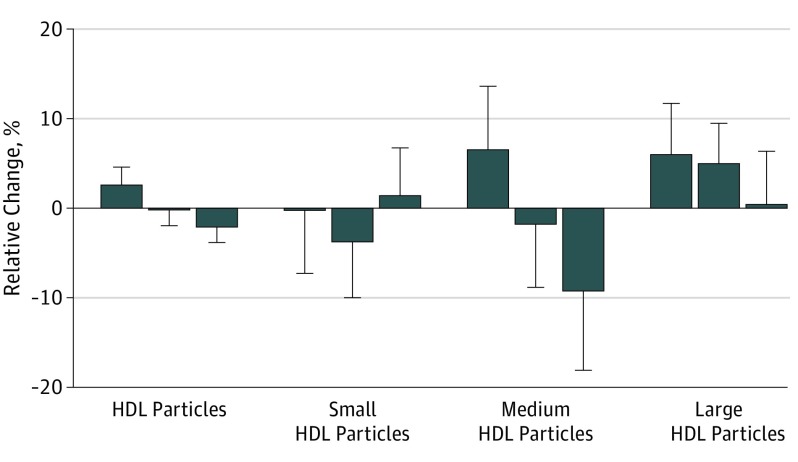
Analyzing the association between tertiles of baseline DMGV levels and total and HDL-P subclass responses to exercise training, both total and medium HDL-P had decreased responses to exercise training across higher DMGV tertiles (total HDL-P mean changes across increasing DMGV tertile: 0.03, −0.002, and −0.02; linear trend P = 9.0 × 10−4; medium HDL-P changes across increasing DMGV tertile: 0.06, −0.01, and −0.09; linear trend P = .009), whereas large HDL-P showed a nonsignificant, attenuated response (Figure 3). In addition, DMGV was associated with changes in insulin sensitivity (β, −0.13; P = 3.0 × 10−3) and body composition (abdominal visceral fat area [centimeters squared], β, 0.11; P = .03; body weight [kilograms], β, 0.11; P = .04) in the minimally adjusted models, but these associations were no longer significant in the fully adjusted model (eTable 6 in the Supplement).
Figure 3. High-Density Lipoprotein (HDL) Particle Total and Subclass Changes After Exercise Training According to Tertile of Baseline Dimethylguanidino Valeric Acid Level.
Increasing tertiles of baseline dimethylguanidino valeric acid level from left to right associated with significant decreases in total and medium HDL particle changes after exercise (β [SE], 0.19 [0.043] and 0.18 [0.045]) and nonsignificant decreases in large HDL particles. Relative change is natural logarithmically transformed final value minus log-transformed initial value.
In exploratory analyses, we tested to see whether asymmetric dimethylarginine levels had similar prognosticative value, since they are a known substrate of DMGV. We found that they were not associated with metabolic health changes after exercise (results not shown).
Discussion
This study has 3 principal findings. First, we extended the spectrum of metabolic risk factors associated with blood levels of DMGV in a young cohort free of cardiometabolic disease. Second, we found regular exercise modulated circulating levels of DMGV and that these changes correlated with the changes in associated clinical traits after exercise training. We then show that higher baseline levels of DMGV were associated with an attenuated response in the number and subclass distribution of HDL-P levels and insulin sensitivity to exercise training. Taken together, these findings highlight the role of DMGV as a very early marker of cardiometabolic disease that is associated with partial resistance to the metabolic health benefits of regular exercise.
Dimethylguanidino valeric acid remains an incompletely understood metabolite with few existing data,18,19 particularly in the context of cardiometabolic diseases, prior to its recent discovery as a marker of hepatic fat and factor associated with incident type 2 diabetes.12 In addition to confirming DMGV’s association with abdominal visceral fat, a closely associated phenotype with liver fat, we found associations with measures of insulin resistance and dyslipidemia after adjusting for body size and visceral fat. These results further support DMGV as a marker of liver fat. Visceral adiposity and hepatic fat are highly interconnected,20 and while each phenotype is associated with metabolic risk factors,21,22,23,24 a growing body of evidence suggests that fat in the liver may confer cardiometabolic risk independent of general and even visceral fat.25,26,27,28 Furthermore, nonalcoholic fatty liver disease has emerged as the most common chronic liver disease in Western populations, and it is increasingly recognized that this disease occurs in individuals without obesity (so-called nonobese or lean fatty liver disease), including white individuals.29 The absence of obesity may lower suspicion for nonalcoholic fatty liver disease and lead to its underdiagnosis.30 Here, we demonstrate DMGV’s close association with visceral adiposity, decreased insulin sensitivity, and dyslipidemia in a young (mean age, 26 years) subsample of HERITAGE participants with normal weight (median BMI, 24). Thus, DMGV may prove useful in the early detection of subclinical metabolic dysfunction in nonobese individuals.
Exercise remains a cornerstone of cardiometabolic disease prevention and treatment, and identifying biomarkers that prognosticate the clinical response to exercise training remains an unmet clinical need. These findings point to DMGV’s value in identifying individuals who may benefit less from regular aerobic exercise in regard to HDL traits and insulin sensitivity. The concept of metabolic resistance to exercise training is not new.4,5,31 Patients with higher BMI were less likely to receive the protective effects of lifestyle or exercise on type 2 diabetes compared with metformin in the Diabetes Prevention Program.1 Other groups have demonstrated that individuals with increased visceral and liver fat were less likely to improve their insulin sensitivity and lipid health after lifestyle interventions that included regular exercise.32,33,34,35 This study differs from these previous ones by highlighting DMGV’s assessment value in a group without apparent metabolic disease. The fact that DMGV is correlated with metabolic dysfunction at baseline and inversely associated with its own responsiveness to exercise training (r = −0.42) raises the possibility of a threshold effect in which patients with higher levels of DMGV require further intervention beyond the HERITAGE endurance exercise program to improve specific metabolic health traits. If validated, these findings could have potential clinical implications, since the HERITAGE exercise protocol (a mean of 126 minutes/week of moderate-intensity to vigorous-intensity exercise, and 150 minutes of vigorous-intensity exercise for the last 6 weeks of the program)15 is similar to current physical activity guideline recommendations (150 minutes/week of moderate-intensity exercise) for improving cardiometabolic health through aerobic exercise.36 Thus, individuals with high levels of DMGV may need alternative exercise programs or additional therapies to improve their lipid profile and insulin sensitivity. Opportunities to further investigate DMGV’s association with exercise interventions are ongoing.11
Interestingly, DMGV remained associated with the responses in several HDL traits to exercise training, including total HDL-P levels, after adjusting for baseline levels of each lipid trait and body mass changes that occurred with exercise. This finding is notable, because total HDL-P level has emerged as an important biomarker of cardiovascular disease risk that may be more strongly associated with outcomes than HDL-C level, apolipoprotein A1, and cholesterol efflux capacity.37,38,39 While pharmacotherapy is associated with increased HDL-P levels and subsequent decreased cardiovascular disease risk,37,40 the effects of regular exercise are less clear, and few studies have examined its effects on total and HDL-P subclass levels in healthy populations. Results from a recent meta-analysis of endurance exercise training’s effects on nuclear magnetic resonance–based HDL subclasses demonstrated increases in large HDL-P levels and decreases in medium HDL-P levels after training.41 In pooled data from 2 small randomized clinical trials of a lifestyle intervention that included diet and/or exercise training in participants with metabolic syndrome, Khan et al42 found that, in comparison with the control group, participants in the exercise arm had significant decreases in small and medium-sized HDL-P levels and nonsignificant increases in large HDL-P levels. The overall increase in HDL-P size correlated with HDL functional improvements,42 although the association between HDL-P subclass and HDL function remains uncertain.43,44,45 Compared with these studies, we found that DMGV levels were associated with the opposite response in HDL-P subclass; namely, DMGV levels were positively correlated with changes in small HDL-P and inversely associated with large HDL-P and HDL-P size changes in the minimally adjusted model, with attenuation of these associations after further adjustment. Prior work has shown that agxt2 variants are associated with triacylglycerol and cholesterol ester concentrations and gene knockdown in a zebrafish model modulated their levels46; however, it remains to be seen if and how DMGV influences HDL metabolism. Future studies in model systems are needed to investigate whether DMGV has functional effects on lipid metabolism.
Limitations
This study has several limitations. The HERITAGE study did not include a control group, and thus any longitudinal changes in clinical and biochemical traits are made in comparison with baseline values. However, HERITAGE used several strategies to quantify the reproducibility of each metabolic trait and their within-person variability both before and after the exercise program to better assess the true effect of regular exercise on these traits.47,48,49 Regular exercise provides numerous health benefits, and although patients with elevated levels of DMGV had attenuated responses in specific metabolic traits, there are likely additional positive effects of this particular training regimen not captured in the study. Nonetheless, identifying biomarkers of targeted health outcomes may benefit specific patient populations. These HERITAGE findings were made exclusively in white participants and may not fully apply to other racial/ethnic groups; however, DMGV levels have been found to prognosticate incident type 2 diabetes in an African American cohort.12
Conclusions
In summary, DMGV is an early marker of metabolic dysfunction associated with diminished responses in HDL traits and insulin sensitivity to endurance exercise training. These findings highlight the potential application of metabolomics to inform targeted exercise therapy. Ongoing studies must also assess whether DMGV or similar compounds contribute in a causal manner to cardiometabolic disease.
eMethods 1. Details of clinical phenotyping in the HERITAGE Family Study
eMethods 2. Details of metabolomics platform
eTable 1. List of clinical phenotypes examined
eTable 2. Associations between baseline DMGV levels and metabolic traits, with and without adjustment for fasting insulin.
eTable 3. Changes in DMGV levels after 20 weeks of exercise training
eTable 4. Associations between changes in DMGV levels and changes in metabolic traits after exercise training
eTable 5. Associations between baseline levels of DMGV and fasting insulin and HDL- trait changes after exercise training.
eTable 6. Changes in metabolic traits according to baseline DMGV levels
eFigure 1. Cross-sectional relationships between baseline DMGV levels and metabolic traits after adjustment for age, sex, BMI, and abdominal visceral fat
eFigure 2. Relationship between baseline DMGV levels and metabolic traits in HERITAGE parents and offspring
eFigure 3. Correlation between baseline DMGV levels and changes in DGMV levels after exercise training
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kraus WE, Houmard JA, Duscha BD, et al. . Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347(19):1483-1492. doi: 10.1056/NEJMoa020194 [DOI] [PubMed] [Google Scholar]
- 3.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159(4):738-749. doi: 10.1016/j.cell.2014.10.029 [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc. 2001;33(6)(suppl):S446-S451. doi: 10.1097/00005768-200106001-00013 [DOI] [PubMed] [Google Scholar]
- 5.de Lannoy L, Clarke J, Stotz PJ, Ross R. Effects of intensity and amount of exercise on measures of insulin and glucose: analysis of inter-individual variability. PLoS One. 2017;12(5):e0177095. doi: 10.1371/journal.pone.0177095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchard C, An P, Rice T, et al. . Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985). 1999;87(3):1003-1008. doi: 10.1152/jappl.1999.87.3.1003 [DOI] [PubMed] [Google Scholar]
- 7.Leon AS, Rice T, Mandel S, et al. . Blood lipid response to 20 weeks of supervised exercise in a large biracial population: the HERITAGE Family Study. Metabolism. 2000;49(4):513-520. doi: 10.1016/S0026-0495(00)80018-9 [DOI] [PubMed] [Google Scholar]
- 8.Boulé NG, Weisnagel SJ, Lakka TA, et al. ; HERITAGE Family Study . Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care. 2005;28(1):108-114. doi: 10.2337/diacare.28.1.108 [DOI] [PubMed] [Google Scholar]
- 9.Bouchard C, Blair SN, Church TS, et al. . Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7(5):e37887. doi: 10.1371/journal.pone.0037887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman NJ. Omics and exercise: global approaches for mapping exercise biological networks. Cold Spring Harb Perspect Med. 2017;7(10):a029884. doi: 10.1101/cshperspect.a029884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruvada P, Laughlin M, McGowan J, et al. . NIH consortium on molecular transducers of physical activity (MoTrPAC). Adv Nutr. 2017;8(1):2-2. [Google Scholar]
- 12.O’Sullivan JF, Morningstar JE, Yang Q, et al. . Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest. 2017;127(12):4394-4402. doi: 10.1172/JCI95995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caplin B, Wang Z, Slaviero A, et al. ; International Consortium for Blood Pressure Genome-Wide Association Studies . Alanine-glyoxylate aminotransferase-2 metabolizes endogenous methylarginines, regulates NO, and controls blood pressure. Arterioscler Thromb Vasc Biol. 2012;32(12):2892-2900. doi: 10.1161/ATVBAHA.112.254078 [DOI] [PubMed] [Google Scholar]
- 14.Perticone F, Sciacqua A, Maio R, et al. . Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46(3):518-523. doi: 10.1016/j.jacc.2005.04.040 [DOI] [PubMed] [Google Scholar]
- 15.Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Gagnon J. The HERITAGE Family Study: aims, design, and measurement protocol. Med Sci Sports Exerc. 1995;27(5):721-729. doi: 10.1249/00005768-199505000-00015 [DOI] [PubMed] [Google Scholar]
- 16.Gagnon J, Province MA, Bouchard C, et al. . The HERITAGE Family Study: quality assurance and quality control. Ann Epidemiol. 1996;6(6):520-529. doi: 10.1016/S1047-2797(96)00068-3 [DOI] [PubMed] [Google Scholar]
- 17.Wilmore JH, Després JP, Stanforth PR, et al. . Alterations in body weight and composition consequent to 20 wk of endurance training: the HERITAGE Family Study. Am J Clin Nutr. 1999;70(3):346-352. doi: 10.1093/ajcn/70.3.346 [DOI] [PubMed] [Google Scholar]
- 18.Martens-Lobenhoffer J, Rodionov RN, Bode-Böger SM. Probing AGXT2 enzyme activity in mouse tissue by applying stable isotope-labeled asymmetric dimethyl arginine as substrate. J Mass Spectrom. 2012;47(12):1594-1600. doi: 10.1002/jms.3125 [DOI] [PubMed] [Google Scholar]
- 19.Rodionov RN, Martens-Lobenhoffer J, Brilloff S, et al. . Role of alanine:glyoxylate aminotransferase 2 in metabolism of asymmetric dimethylarginine in the settings of asymmetric dimethylarginine overload and bilateral nephrectomy. Nephrol Dial Transplant. 2014;29(11):2035-2042. doi: 10.1093/ndt/gfu236 [DOI] [PubMed] [Google Scholar]
- 20.Kabir M, Catalano KJ, Ananthnarayan S, et al. . Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2005;288(2):E454-E461. doi: 10.1152/ajpendo.00203.2004 [DOI] [PubMed] [Google Scholar]
- 21.Fox CS, Massaro JM, Hoffmann U, et al. . Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39-48. doi: 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Musani SK, Bidulescu A, et al. . Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224(2):521-525. doi: 10.1016/j.atherosclerosis.2012.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369-1375. doi: 10.1053/j.gastro.2008.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim LJ, Nalls MA, Eiriksdottir G, et al. ; AGES-Reykjavik Study Investigators . Associations of visceral and liver fat with the metabolic syndrome across the spectrum of obesity: the AGES-Reykjavik study. Obesity (Silver Spring). 2011;19(6):1265-1271. doi: 10.1038/oby.2010.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HJ, Kim HJ, Lee KE, et al. . Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164(19):2169-2175. doi: 10.1001/archinte.164.19.2169 [DOI] [PubMed] [Google Scholar]
- 26.Targher G, Bertolini L, Rodella S, et al. . NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring). 2008;16(6):1394-1399. doi: 10.1038/oby.2008.64 [DOI] [PubMed] [Google Scholar]
- 27.Fabbrini E, Magkos F, Mohammed BS, et al. . Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106(36):15430-15435. doi: 10.1073/pnas.0904944106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter SA, Pedley A, Massaro JM, Vasan RS, Hoffmann U, Fox CS. Aminotransferase levels are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33(1):139-146. doi: 10.1161/ATVBAHA.112.300075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D, Kim WR. Nonobese Fatty Liver Disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485. doi: 10.1016/j.cgh.2016.08.028 [DOI] [PubMed] [Google Scholar]
- 30.Cusi K. Nonalcoholic steatohepatitis in nonobese patients: not so different after all. Hepatology. 2017;65(1):4-7. doi: 10.1002/hep.28839 [DOI] [PubMed] [Google Scholar]
- 31.Slentz CA, Bateman LA, Willis LH, et al. . Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab. 2011;301(5):E1033-E1039. doi: 10.1152/ajpendo.00291.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thamer C, Machann J, Stefan N, et al. . High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity (Silver Spring). 2007;15(2):531-538. doi: 10.1038/oby.2007.568 [DOI] [PubMed] [Google Scholar]
- 33.Johnson NA, Sachinwalla T, Walton DW, et al. . Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50(4):1105-1112. doi: 10.1002/hep.23129 [DOI] [PubMed] [Google Scholar]
- 34.Keating SE, Hackett DA, Parker HM, et al. . Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63(1):174-182. doi: 10.1016/j.jhep.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 35.Hallsworth K, Thoma C, Hollingsworth KG, et al. . Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci (Lond). 2015;129(12):1097-1105. doi: 10.1042/CS20150308 [DOI] [PubMed] [Google Scholar]
- 36.US Department of Health and Human Services 2008. Physical activity guidelines for Americans. https://health.gov/paguidelines/2008/pdf/paguide.pdf. Published 2008. Accessed April 18, 2019.
- 37.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128(11):1189-1197. doi: 10.1161/CIRCULATIONAHA.113.002671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackey RH, Greenland P, Goff DC Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2012;60(6):508-516. doi: 10.1016/j.jacc.2012.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khera AV, Demler OV, Adelman SJ, et al. . Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. 2017;135(25):2494-2504. doi: 10.1161/CIRCULATIONAHA.116.025678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otvos JD, Collins D, Freedman DS, et al. . Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113(12):1556-1563. doi: 10.1161/CIRCULATIONAHA.105.565135 [DOI] [PubMed] [Google Scholar]
- 41.Sarzynski MA, Burton J, Rankinen T, et al. . The effects of exercise on the lipoprotein subclass profile: a meta-analysis of 10 interventions. Atherosclerosis. 2015;243(2):364-372. doi: 10.1016/j.atherosclerosis.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan AA, Mundra PA, Straznicky NE, et al. . Weight loss and exercise alter the high-density lipoprotein lipidome and improve high-density lipoprotein functionality in metabolic syndrome. Arterioscler Thromb Vasc Biol. 2018;38(2):438-447. doi: 10.1161/ATVBAHA.117.310212 [DOI] [PubMed] [Google Scholar]
- 43.Du XM, Kim MJ, Hou L, et al. . HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116(7):1133-1142. doi: 10.1161/CIRCRESAHA.116.305485 [DOI] [PubMed] [Google Scholar]
- 44.Fazio S, Pamir N. HDL particle size and functional heterogeneity. Circ Res. 2016;119(6):704-707. doi: 10.1161/CIRCRESAHA.116.309506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarzynski MA, Ruiz-Ramie JJ, Barber JL, et al. . Effects of increasing exercise intensity and dose on multiple measures of HDL (high-density lipoprotein) function. Arterioscler Thromb Vasc Biol. 2018;38(4):943-952. doi: 10.1161/ATVBAHA.117.310307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhee EP, Ho JE, Chen MH, et al. . A genome-wide association study of the human metabolome in a community-based cohort. Cell Metab. 2013;18(1):130-143. doi: 10.1016/j.cmet.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Després JP, Gagnon J, Bergeron J, et al. . Plasma post-heparin lipase activities in the HERITAGE Family Study: the reproducibility, gender differences, and associations with lipoprotein levels. health, risk factors, exercise training and genetics. Clin Biochem. 1999;32(3):157-165. doi: 10.1016/S0009-9120(98)00106-4 [DOI] [PubMed] [Google Scholar]
- 48.Daw EW, Province MA, Gagnon J, et al. . Reproducibility of the HERITAGE Family Study intervention protocol: drift over time. Ann Epidemiol. 1997;7(7):452-462. doi: 10.1016/S1047-2797(97)00082-3 [DOI] [PubMed] [Google Scholar]
- 49.Skinner JS, Wilmore KM, Jaskolska A, et al. . Reproducibility of maximal exercise test data in the HERITAGE Family Study. Med Sci Sports Exerc. 1999;31(11):1623-1628. doi: 10.1097/00005768-199911000-00020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Details of clinical phenotyping in the HERITAGE Family Study
eMethods 2. Details of metabolomics platform
eTable 1. List of clinical phenotypes examined
eTable 2. Associations between baseline DMGV levels and metabolic traits, with and without adjustment for fasting insulin.
eTable 3. Changes in DMGV levels after 20 weeks of exercise training
eTable 4. Associations between changes in DMGV levels and changes in metabolic traits after exercise training
eTable 5. Associations between baseline levels of DMGV and fasting insulin and HDL- trait changes after exercise training.
eTable 6. Changes in metabolic traits according to baseline DMGV levels
eFigure 1. Cross-sectional relationships between baseline DMGV levels and metabolic traits after adjustment for age, sex, BMI, and abdominal visceral fat
eFigure 2. Relationship between baseline DMGV levels and metabolic traits in HERITAGE parents and offspring
eFigure 3. Correlation between baseline DMGV levels and changes in DGMV levels after exercise training