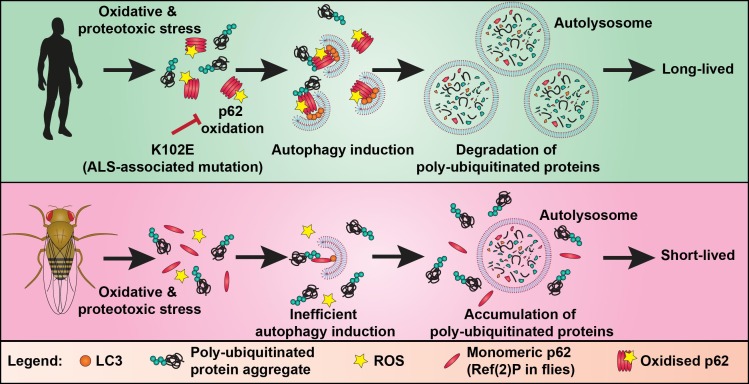
Figure 1. FIGURE 1: Diagram of the proposed evolutionary adaptation to the autophagy machinery involving oxidation of p62.
Oxidative and proteotoxic stress triggers oxidation and oligomerisation of p62, promoting the formation of autophagosomes around poly-ubiquitinated proteins. After the autophagosome is fully formed it fuses with a lysosome to form the autolysosome. In the autolysosome, the cargo (e.g. poly-ubiquitinated protein aggregates and p62) is degraded by lysosomal proteases. The ALS-associated mutation K102E can impair oxidation of p62, resulting in less autophagy induction in oxidative and proteotoxic stress conditions. The key cysteine residues required for p62 oxidation, present in vertebrates, were acquired late in evolution. Ref(2)P, the fly homolog of p62, lacks these oxidation-sensitive cysteine residues. Introduction of the p62 redox-sensitive cysteines into Ref(2)P promotes autophagy and survival in oxidative and proteotoxic stress conditions. This suggests that longevity associated with vertebrates, like humans, is a result of evolutionary adaptations to their autophagy machinery, which allow them to better manage the age-related increase in proteotoxic burden.