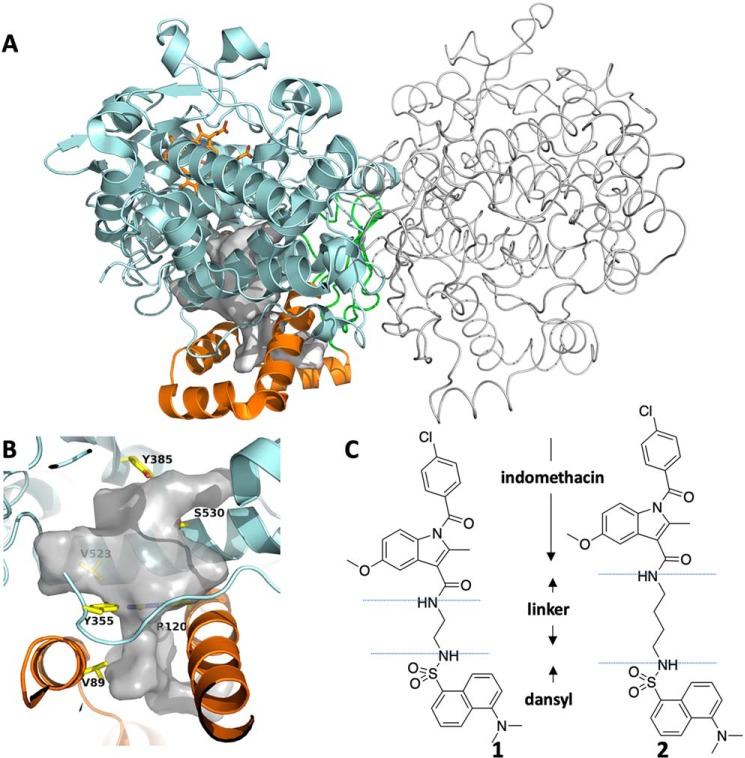
Figure 1.
The structures of cyclooxygenase-2, compound 1, and compound 2. A, the homodimer structure of COX-2 with one monomer represented in cartoon format and the other in tube format. The EGF domain is colored green, the MBD is orange, and the catalytic domain is cyan. Heme is shown in orange ball-and-stick. B, closeup of the active site of COX-2. The void space in the COX-2 active site and lobby region is colored white, and key residues are represented in yellow sticks. These include Tyr-385, site of the catalytic tyrosyl radical; Ser-530, site of inactivation by aspirin acetylation; constriction residues Tyr-355 and Arg-120; and Val-89, which plays a role in MBD structure and inhibitor binding kinetics. C, the chemical structures of compounds 1 and 2.