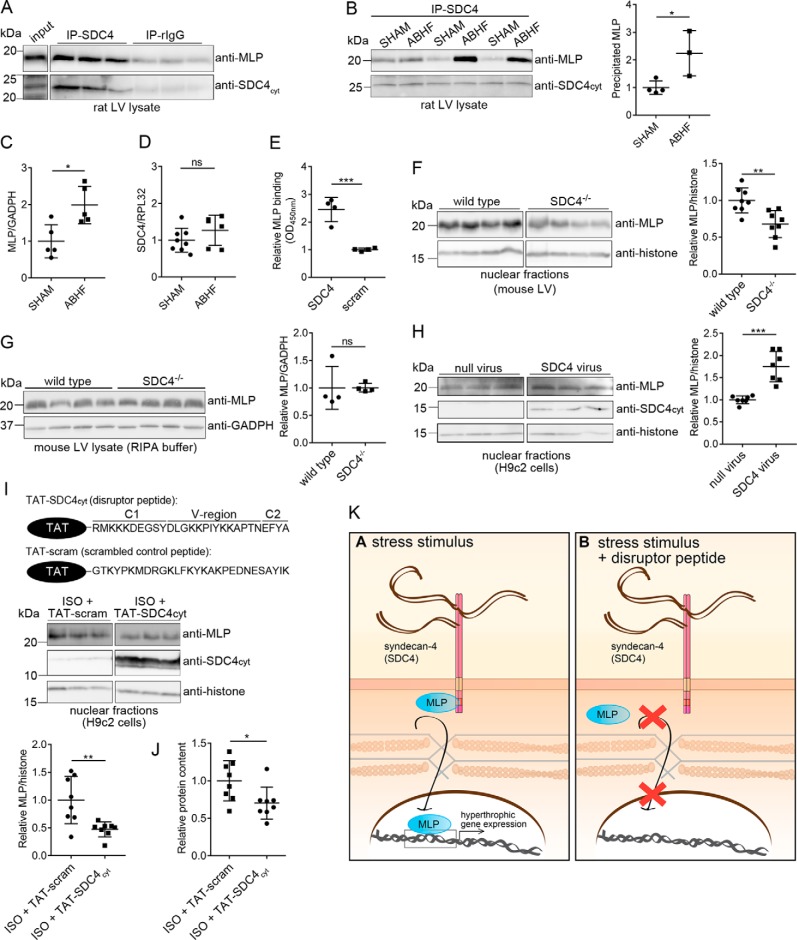
Figure 6.
Syndecan-4 binds directly to MLP and mediates its nuclear translocation. LV lysates from WT rat (A) and ABHF or SHAM rats (six individual rat hearts) (B) were subjected to immunoprecipitation using anti-syndecan-4 (KY/8.2, which has an extracellular epitope). Levels of immunoprecipitated MLP and syndecan-4 were analyzed by immunoblotting with anti-MLP and anti-syndecan-4cyt (which has an intracellular epitope). Nonrelevant rat IgG was used as a negative control. C, total MLP protein level in SHAM and ABHF lysates was analyzed through semiquantified densitometry analysis of immunoblots shown in Fig. S5A. D, syndecan-4 expression in SHAM and ABHF was analyzed by qPCR. E, recombinant MLP bound directly to syndecan-4 in an ELISA-based assay. A scrambled peptide was used as a negative control. Immunoblot analysis of MLP levels in nuclear-enriched fractions (F) and RIPA extracts of LV are from WT and syndecan-4−/− mice (G). Immunoblot analysis of MLP levels in H9c2 cells transduced with a syndecan-4 adenovirus or null virus (H), and H9c2 cells treated with a disruptor peptide (TAT–SDC4cyt) or a scrambled control peptide (TAT-scram) in presence of ISO (I) are shown. The ∼10–15-kDa syndecan-4 band represents the intracellular fragment after shedding of the extracellular domain and is visible when overexpressing syndecan-4 (52). Histone H3 was used as a loading marker for the nuclear-enriched fractions in F, H, and I. B and F–I, scatter plots show MLP levels semiquantified by densitometry analysis. J, protein content in cytoplasmic fractions of H9c2 cells treated with TAT–SDC4cyt and TAT-scram control peptide (n = 8). K, schematic illustration of the disruptor peptide experiment. Differences were tested using unpaired t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001, ns indicates not significant) (n = 3–8). Error bars, S.D.