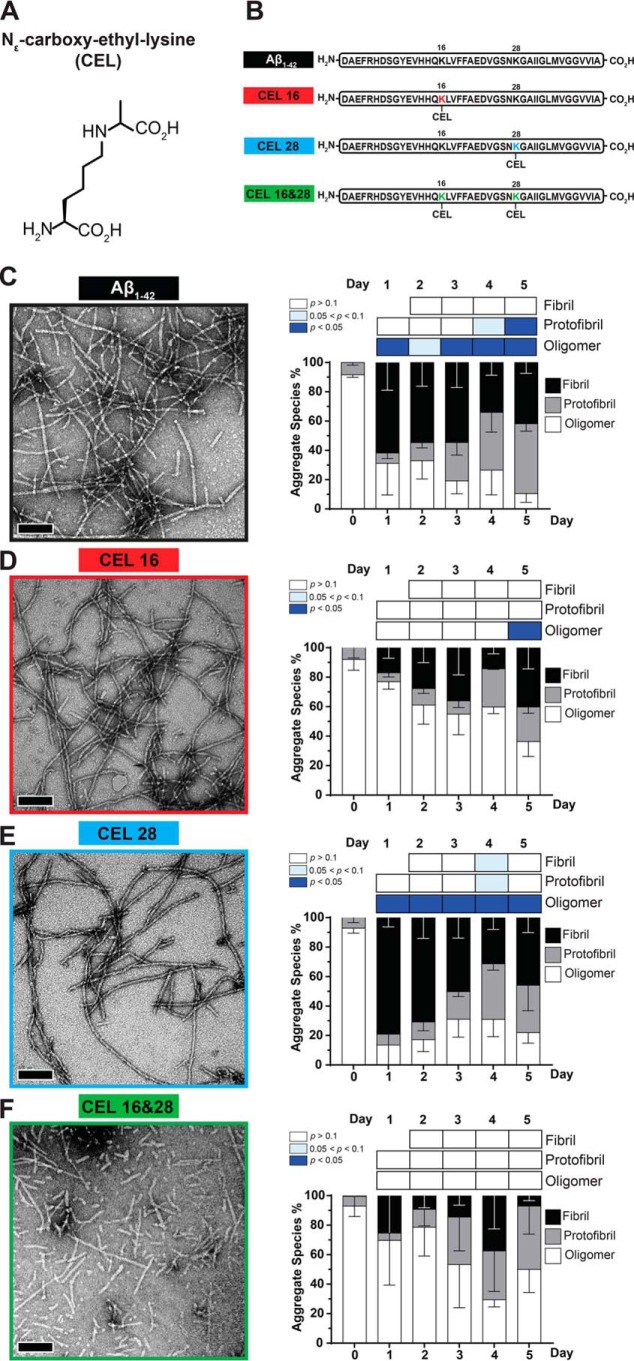
Figure 1.
Double-CEL modifications at Lys-16 and Lys-28 differentially affect Aβ1–42 aggregation over 5 days. A, chemical structure of CEL. B, peptide sequences of Aβ1–42 and the three CEL variants on Aβ1–42. C–F, Aβ-CEL16&28 do not form extensive fibrils and have a high abundance of protofibrils and oligomers after 5 days in a fibril-promoting buffer relative to all CEL-glycated peptides and unglycated Aβ1–42. Representative TEM micrographs at day 5 (left panel) (n = 2–3; scale bar = 100 nm) with the graph of % aggregate species (right panel) showing proportions of fibrils, protofibrils, and oligomers over 5 days. *, p < 0.05. p values for oligomers and protofibrils are relative to day 0 and fibrils are relative to day 1 using Bonferroni's post hoc test in a one-way analysis of variance (ANOVA). See Fig. S1 for synthesis of peptide and Fig. S2 for examples of fibrils, protofibrils, and oligomers.