Abstract
Background:
Hemodialysis (HD) is the main form of renal replacement therapy available in Nigeria. However, this is still largely unaffordable by individuals with resultant poor outcomes.
Methods:
This was a retrospective study of all patients with renal failure who had dialysis in the renal unit of Dalhatu Araf Specialist Hospital over the past 2 years. Information retrieved included sex, age, cause of renal failure, human immunodeficiency virus status, hepatitis B surface antigen status, antibodies to hepatitis C virus status, number of sessions, total duration on dialysis (in weeks), use of erythropoietin (EPO), common problems encountered on the dialysis machine, and the outcome of the patient.
Results:
A total of 68 patients (50% males) were enrolled in the study. The mean age was 41 ± 15 years (17–75), and mean weight in kilograms was 64.3 ± 10.9 (42–87). Acute kidney injury was seen in 18 (26.5%), while 50 (73.5%) had end-stage renal disease (ESRD). Chronic glomerulonephritis was the leading cause of ESRD (46%) with autosomal dominant polycystic kidney disease being the least (2%). The mean packed cell volume at the start of dialysis was 25.7% ± 5.9%. Tunneled necklines were in 11 (16.8%) and femoral catheters were in 48 (70.6%). The median total number of sessions was 4.0 (1–136), while the median duration on dialysis was 1 week (1–48) with both sexes having the same duration on dialysis (P = 0.44). The average frequency of dialysis among those with ESRD was twice weekly. Only 15 (30.0%) of those with ESRD continued dialysis after 3 months. The median survival time for females was 5 weeks while that for the males was 20 weeks (P = 0.108). EPO use was in 12 (17.7%) being 4000 IU once weekly. Cramps complicated the first sessions of dialysis in 27 (39.7%) patients.
Conclusion:
The survival of patients on HD in our environment is poor due largely to poor affordability despite its availability.
Keywords: Hemodialysis, mortality, outcomes, practice, resource poor
INTRODUCTION
Renal replacement therapy has revolutionized the management of renal failure patients worldwide with hemodialysis (HD) being the main modality practiced in many countries in the sub-Saharan subregion1,2. In Nigeria, HD has been the mainstay of renal replacement therapy since its inception in the 1980s.3,4 In an attempt to meet the need of the increasing number of patients with renal failure in the country, there has been a proliferation of HD units all over the country with majority concentrated around the big cities. World over, standard guidelines recommend a minimum maintenance dialysis dose of three sessions a week, each of a duration of 3–5 h5,6,7,8 which should ensure dialysis adequacy. However, the practice of HD in Nigeria is a far cry from the recommended standards of care as HD practice in Nigeria is suboptimal despite the ever-increasing number of centers. Studies conducted over a decade ago in north central and southwestern Nigeria showed that HD practice is grossly inadequate among end-stage renal disease (ESRD) patients where the majority of patients within the subregion had less than three sessions of dialysis per week.9,10 Recent studies in Nigeria have not demonstrated a change in trend.11,12 Factors responsible for this ranges from poor health financing, affordability due to out-of-pocket payment and grossly inadequate HD centers in the country.13,14
Suboptimal dialysis in countries within the subregions such as Ghana and Ethiopia including Nigeria results in poor outcomes as evidenced by high mortality and morbidity among ESRD patients.15,16,17 Dropout rate from dialysis could be as high as 90% after 90 days on maintenance dialysis for patients with ESRD within the subregion compared to <20% in the developed world.1 Vascular access for HD is also another impediment to HD practice as the use of femoral vein catheterization as vascular access for HD whether for acute or chronic cases which is obsolete in the resource buoyant settings18 is the major route of access in resource-constrained regions11,14,19 with its attendant increased risk for vascular access infections and even deaths.20
In this study, we performed a 2-year review of HD at a tertiary health center in north central Nigeria with regard to the causes of renal failure, the average duration of dialysis before drop out, the types of vascular accesses employed, and the common complications encountered during dialysis.
METHODS
This was a retrospective study of all patients who had dialysis for renal failure in the renal unit of Dalhatu Araf Specialist Hospital, Lafia, over a 2-year period from January 2014 to December 2015. Information retrieved from patient case notes and dialysis registers included age, sex, cause and type of renal failure, viral serologies (human immunodeficiency virus, hepatitis B surface antigen, and antihepatitis C virus antibodies), packed cell volume (PCV) at presentation, type of dialysis access employed, frequency of dialysis, number of dialysis sessions, duration of dialysis, and common problems encountered during dialysis.
Ethical clearance was obtained from the Health Research Ethics Committee of Dalhatu Araf Specialist hospital, Lafia.
Data were analyzed using the Epi Info 7.1 statistical software (CDC, Atlanta, GA). Mean ± standard deviation was used to describe normally distributed continuous variables and proportions for categorical variables. Median with interquartile range was used to describe nonnormally distributed continuous variables. The Student's t-test was used to compare group means and the Chi-square test to compare proportions. The fisher's exact was used where cells contained <5 observations in cross-tabulations. The Kaplan–Meier graph was used to determine the survival probability of the study population. We considered P < 0.05 as statistically significant.
RESULTS
A total of 68 patients had dialysis over the period of two reviews, 34 (50%) being males. The mean age was 41 ± 15 years, and mean weight in kilograms was 64.3 ± 10.9. The mean PCV at the start of dialysis was 25.7% ± 5.9% as shown in Table 1.
Table 1.
Characteristics of patients dialyzed at the Dalhatu Araf Specialist Hospital, Lafia, between January 2014 and December 2015
| Variable | Total (n=68) (%) | Female | Male | P |
|---|---|---|---|---|
| Age (years) | 41±15 (17-75) | 40±15 | 42±16 | 0.57 |
| Weight (kg), (n=60) | 64.2±10.8 | 64.0±12.5 | 64.4±9.3 | 0.87 |
| Pre-HD PCV (%) | 25.7±5.9 | 25.1±5.8 | 26.4±6.1 | 0.36 |
| HIV | 10 (14.7) | 7 (10.3) | 3 (4.4) | 0.15 |
| HBsAg | 1 (1.5) | 1 (1.5) | 0 (0.0) | 0.5 |
| Anti-HCV antibodies | 5 (7.4) | 0 (0.0) | 5 (7.4) | 0.02 |
| Femoral catheter | 48 (70.5) | 25 (73.5) | 23 (67.7) | 0.79 |
| Permanent catheter | 11 (16.1) | 5 (14.7) | 6 (17.6) | 0.74 |
| Temporary internal jugular line | 7 (10.2) | 4 (11.7) | 3 (8.8) | 1.000* |
| Arteriovenous fistula | 2 (2.9) | 0 (0.0) | 2 (5.8) | 0.49* |
| Median total number of sessions | 4.0 (1-136) | 4 (1-45) | 4 (1-136) | 0.75 |
| Median total duration in weeks | 1 (1-48) | 1 (1-28) | 1 (1-48) | 0.44 |
| Use of erythropoietin | 12 (17.6) | 4 (5.9) | 8 (11.7) | 0.17 |
| ESRD (n=50) | ||||
| CGN | 23 (46.0) 10 (20.0) | 13 (46.4) | 10 (45.4) | 0.94 |
| DM | 8 (16.0) | 6 (21.4) | 4 (18.1) | 1.000* |
| HTN | 6 (12.0) | 3 (10.7) | 5 (22.7) | 0.27* |
| OBS URO | 2 (4.0) | 4 (14.2) | 2 (9.0) | 0.68* |
| HIVAN | 1 (2.0) | 1 (3.5) | 1 (4.5) | 1.000* |
| ADPKD | 1 (3.5) | 0 (0.0) | 1.000* | |
| AKI (n=18) | ||||
| Sepsis | 5 (27.7) | 1 (16.6) | 4 (33.3) | 0.60* |
| PPH | 4 (22.2) | 0 (0.0) | 4 (33.3) | 0.24* |
| AGN | 2 (11.1) | 1 (16.6) | 1 (8.3) | 1.000* |
| Malignant HTN | 1 (5.5) | 1 (16.6) | 0 (0.0) | 0.33* |
| Dehydration | 1 (5.5) | 1 (16.6) | 0 (0.0) | 0.33* |
| Others | 5 (27.7) | 2 (33.3) | 3 (25.0) | 1.000* |
*Fisher’s exact test, others (drug toxicity in HIV). Pre-HD PCV – Predialysis packed cell volume; HD: Hemodialysis; HIV – Human immunodeficiency virus; HBsAg – Hepatitis B surface antigen; Anti-HCV antibodies – Antibodies to hepatitis C virus; ESRD – End-stage renal disease; CGN – Chronic glomerulonephritis; DM – Diabetes mellitus; HTN – Hypertension, OBS URO – Obstructive uropathy, HIVAN – HIV-associated nephropathy; ADPKD – Autosomal dominant polycystic kidney disease; AKI – Acute kidney injury; PPH – Postpartum hemorrhage; AGN – Acute glomerulonephritis
Acute kidney injury (AKI) was seen in 18 (26.5%) with sepsis being the major cause while 50 (73.5%) had ESRD. Chronic glomerulonephritis (CGN) was the leading cause of ESRD in 23 patients (46%) followed closely by hypertension. None of the ESRD patients had pre-ESRD care by a nephrologist before needing dialysis.
Femoral catheters were inserted in 48 (70.6%) patients and tunneled (permanent catheters) necklines in 11 (16.2%) while only 2 (2.9%) patients dialyzed through A-V fistula.
The median total number of sessions was 4.0 (1–136), while the median duration on dialysis was 1 week (1–48) being similar in both sexes (P = 0.44). The average frequency of dialysis among those with ESRD was twice weekly, while erythropoietin (EPO) use was in 12 (24%) being 4000 IU once weekly among those with ESRD.
Complications experienced during dialysis included vomiting in 5 (7.6%), cramps 27 (41.5%), hypotension 15 (23.0%), and fever 27 (41.5%).
Of the 18 patients with AKI, 6 (33.3%) recovered fully and another 6 died while the remaining 6 were lost to follow-up. Twenty-eight (57%) of the ESRD patients died during the period of the study while 13 (26%) were lost to follow-up. Only 15 (30.0%) of those with ESRD continued dialysis after 3 months of initiation. Using the log-rank test, males had a longer median survival time of 20 weeks (95% confidence interval, 1.60–38.40) compared to their female counterparts who had 5 weeks (95%, 1.28–8.73) with no significant difference between them, P = 0.108 as shown in Table 2 and Figure 1.
Table 2.
Median survival characteristics of the hemodialysis patients
| Variable | Median survival time (weeks) | 95% CI | P |
|---|---|---|---|
| Female | 5 | 1.28-8.73 | 0.108a |
| Male | 20 | 1.60-38.40 |
aLog-rank (mantel cox) test; CI – Confidence interval
Figure 1.
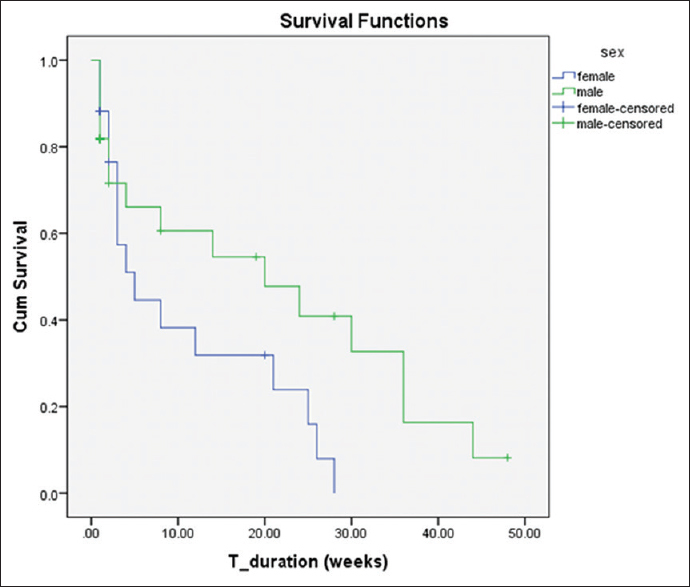
The survival probability of the male and female participants who had hemodialysis in Dalhatu Araf Specialist Hospital
DISCUSSION
In this study, we describe a 2-year experience of the practice of HD in an upcoming center in central Nigeria. The most common cause of AKI requiring dialysis was sepsis while CGN was the most common cause of ESRD in patients dialyzed. Temporary vascular accesses were the most commonly used access for dialysis. The average patient dialyzed only once a week with the total number of dialysis sessions being four. Only 30% of ESRD patients continued dialysis beyond 3 months, and the survival probability established that nearly all patients (with ESRD) will not survive beyond 7 months.
CGN as a leading cause of ESRF in our study was similar to that reported in previous studies in Nigeria and Ghana.11,14,15,17 However, this differs from studies from Ethiopia as well as some developed countries as reported in the Dialysis Outcomes and Practice Patterns Study where diabetes nephropathy was the leading cause of renal failure.16,21 However, our study confirms the increasing contribution of DM to ESRD. Our findings on the leading causes of AKI were similar to those reported by studies in southern Nigeria.11,22 Uchino et al. also reported that sepsis was the leading contributing factor to AKI in critically ill patients in countries from Europe, the Americas, and Asia.23
Central vein catheter insertion through the femoral vein as a predominant route for vascular access in our study is similar to the current practice in Nigeria;11,14 however, this is different from what obtains in Ethiopia, East Africa, where catheter use is about 41%.16 Even though the femoral vein has been the predominant route of vascular access in Nigeria, unpublished report in Jos, north central Nigeria, shows an increasing rate of use of nontunneled and permanent (tunneled) catheter use through the internal jugular vein: a trend shown in this study. In our study, an abysmally low proportion of ESRD patients had A-V fistula as a form of vascular access which is in contrast to HD practice in Ethiopia where the rates are up to 45%16 and between 67% and 91% in the UK, Australia, Japan, and some countries in Europe.24
As a result of out-of-pocket payment for dialysis services, dialysis frequency for majority of our patients was poor. This scenario is similar to the findings previously reported in Nigeria.14,17 Expectedly, the survival outcome for our patients was grim with only a third able to continue dialysis at 3 months and nearly all dead by 7 months. This pattern is similar to previous reported studies14,17 in Nigeria. Interestingly, the studies in Ghana and Ethiopia reported better survival rates at 90 days on HD even though these countries share similar or worse economic indices than Nigeria.15,16 Several reports have established that mortality is highest during the first 90–120 days of commencement of HD.20,25,26 The reasons advanced for this include the use of catheters for vascular access, low serum albumin levels in the patients, advanced age, and withdrawal from dialysis among others. Bradbury et al.26 also found that the presence of pre-ESRD care was associated with a reduction in mortality within the first 120 days and suggested the need for an early contact with a nephrologist at least a month before initiation of HD. The reasons for the high mortality in our study stem from the fact that almost all our patients were “gate crashers” with no pre-ESRD care, and nearly, all of them had catheters for vascular access. Furthermore, out-of-pocket payment contributed to the high rate of discontinuation from dialysis.
This study was limited by the retrospective design as missing and incomplete data are common with this design. Second, this study had a relatively small sample size. We also did not look at other established factors that could contribute to early mortalities such as low serum albumin and phosphate and comorbid factors such as heart failure and cancers.26
CONCLUSION
The survival of patients on HD is poor in our environment as the majority of ESRD patients cannot afford maintenance HD. There is the urgent need for government and other spirited agencies to fund renal replacement therapies. The need for early detection of chronic kidney disease (CKD) and institution of pre-ESRD care as well as measures to slow CKD progression is imperative.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors appreciate the efforts of the renal nurses of the dialysis unit of the hospital who collected the data over the period of the study.
REFERENCES
- 1.Grassmann A, Gioberge S, Moeller S, B G. ESRD patients in 2004: Global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant. 2005;20:2587–93. doi: 10.1093/ndt/gfi159. [DOI] [PubMed] [Google Scholar]
- 2.Naicker S. End-stage renal disease in Sub-Saharan Africa. Ethn Dis. 2009;19:S1. [PubMed] [Google Scholar]
- 3.Bamgboye EL, Mabayoje MO, Odutola TA, Mabadeje AF. Acute renal failure at the Lagos university teaching hospital: A 10-year review. Ren Fail. 1993;15:77–80. [PubMed] [Google Scholar]
- 4.Alebiosu CO, Ayodele OO, Abbas A, Olutoyin AI. Chronic renal failure at the Olabisi Onabanjo university teaching hospital, Sagamu, Nigeria. Afr Health Sci. 2006;6:132–8. doi: 10.5555/afhs.2006.6.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan CT, Blankestijn PJ, Dember LM, Gallieni M, Harris DCH, Lok CE, et al. Dialysis initiation, modality choice, access, and prescription: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019:pii: S0085-2538(19)30138-3. doi: 10.1016/j.kint.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Gray NA, Dent H, McDonald SP. Renal replacement therapy in rural and urban Australia. Nephrol Dial Transplant. 2012;27:2069–76. doi: 10.1093/ndt/gfr584. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66:884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Pippias M, Stel VS, Abad Diez JM, Afentakis N, Herrero-Calvo JA, Arias M, et al. Renal replacement therapy in Europe: A summary of the 2012 ERA-EDTA registry annual report. Clin Kidney J. 2015;8:248–61. doi: 10.1093/ckj/sfv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agaba EI, Lopez A, Ma I, Martinez R, Tzamaloukas RA, Vanderjagt DJ, et al. Chronic hemodialysis in a Nigerian teaching hospital: Practice and costs. Int J Artif Organs. 2003;26:991–5. doi: 10.1177/039139880302601104. [DOI] [PubMed] [Google Scholar]
- 10.Arije A, Kadiri S, Akinkugbe OO. The viability of hemodialysis as a treatment option for renal failure in a developing economy. Afr J Med Med Sci. 2000;29:311–4. [PubMed] [Google Scholar]
- 11.Ekrikpo UE, Udo AI, Ikpeme EE, Effa EE. Haemodialysis in an emerging centre in a developing country: A two year review and predictors of mortality. BMC Nephrol. 2011;12:50. doi: 10.1186/1471-2369-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bello BT, Raji YR, Sanusi I, Braimoh RW, Amira OC, Mabayoje OM. Challenges of providing maintenance hemodialysis in a resource poor country: Experience from a single teaching hospital in Lagos, Southwest Nigeria. Hemodial Int. 2013;17:427–33. doi: 10.1111/hdi.12024. [DOI] [PubMed] [Google Scholar]
- 13.Agbaji OO, Abene EE. Care of patients with end-stage renal disease in Nigeria : A call for a change in paradigm. Jos J Med. 2012;6:28–31. [Google Scholar]
- 14.Oluyombo R, Okunola OO, Olanrewaju TO, Soje MO, Obajolowo OO, Ayorinde MA. Challenges of hemodialysis in a new renal care center: Call for sustainability and improved outcome. Int J Nephrol Renovasc Dis. 2014;7:347–52. doi: 10.2147/IJNRD.S65835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eghan BA, Amoako-Atta K, Kankam CA, Nsiah-Asare A. Survival pattern of hemodialysis patients in Kumasi, Ghana: A summary of forty patients initiated on hemodialysis at a new hemodialysis unit. Hemodial Int. 2009;13:467–71. doi: 10.1111/j.1542-4758.2009.00379.x. [DOI] [PubMed] [Google Scholar]
- 16.Shibiru T, Gudina EK, Habte B, Derbew A, Agonafer T. Survival patterns of patients on maintenance hemodialysis for end stage renal disease in Ethiopia: Summary of 91 cases. BMC Nephrol. 2013;14:127. doi: 10.1186/1471-2369-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makusidi MA, Liman HM, Yakubu A, Isah MD, Abdullahi S, Chijioke A. Hemodialysis performance and outcomes among end stage renal disease patients from Sokoto, North-Western Nigeria. Indian J Nephrol. 2014;24:82–5. doi: 10.4103/0971-4065.127889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, et al. Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int. 2002;61:305–16. doi: 10.1046/j.1523-1755.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 19.Ekpe EE, Ekirikpo U. Challenges of vascular access in a new dialysis centre – Uyo experience. Pan Afr Med J. 2010;7:23. [PMC free article] [PubMed] [Google Scholar]
- 20.Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol. 2011;6:2642–9. doi: 10.2215/CJN.03680411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young EW, Dykstra DM, Goodkin DA, Mapes DL, Wolfe RA, Held PJ. Hemodialysis vascular access preferences and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2002;61:2266–71. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 22.Okunola OO, Ayodele OE, Adekanle AD. Acute kidney injury requiring hemodialysis in the tropics. Saudi J Kidney Dis Transpl. 2012;23:1315–9. doi: 10.4103/1319-2442.103587. [DOI] [PubMed] [Google Scholar]
- 23.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 24.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, et al. Vascular access use and outcomes: An international perspective from the dialysis outcomes and practice patterns study. Nephrol Dial Transplant. 2008;23:3219–26. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson BM, Zhang J, Morgenstern H, Bradbury BD, Ng LJ, McCullough KP, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85:158–65. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, et al. Predictors of early mortality among incident US hemodialysis patients in the dialysis outcomes and practice patterns study (DOPPS) Clin J Am Soc Nephrol. 2007;2:89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]


