Figure 7.
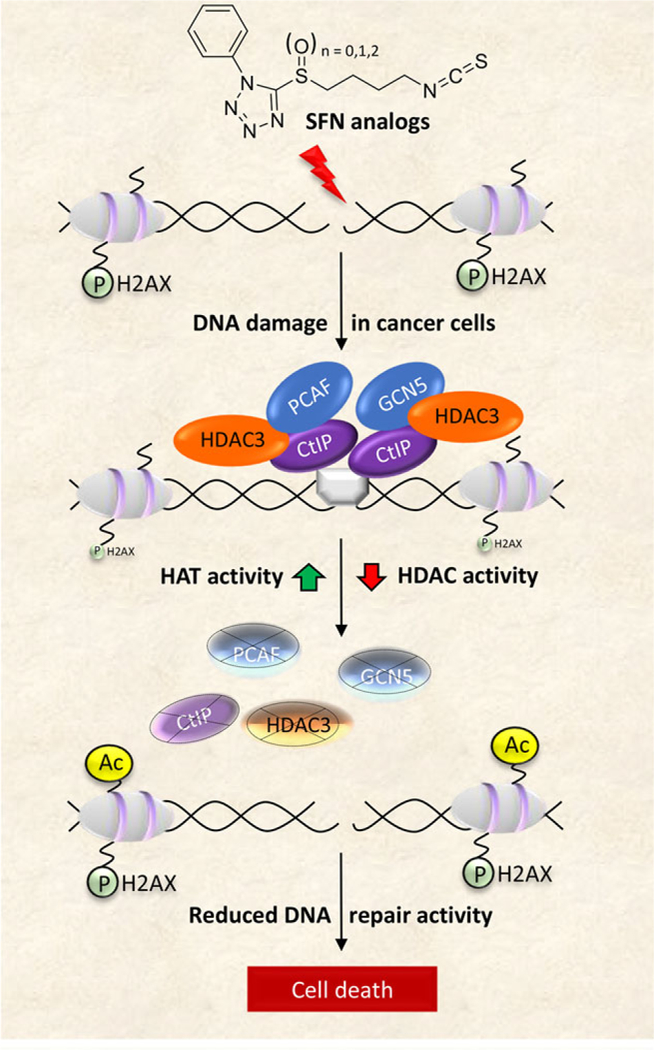
Working model of HDAC/HAT modulation by SFN analogs impacting DNA repair pathways, leading to cancer cell death. Ac, acetylation; P, phosphorylation. Through various mechanisms, not well characterized, SFN analogs trigger DSB in DNA that become flanked by increased pH2AX levels. A functional DNA damage response requires the formation of an efficient repair complex, typically comprising an MRN complex (gray box bridging the DNA break point) bound by the DNA resection and repair factor, CtIP. HATs and HDACs, such as PCAF, GCN5, and HDAC3, regulate the acetylation status of CtIP and maintain its activity/stability, leading to efficient DNA repair and reduced pH2AX levels. Treatment with SFN analogs causes an imbalance in HAT/HDAC activity and protein expression, leading to increased histone acetylation. This also adversely affects the stability of CtIP and its binding partners, including PCAF, GCN5, and HDAC3. As a crucial factor for DNA end resection, reduced levels of CtIP negatively influence homologous recombination (HR), concluding in impairment of DSB repair and eventual cell death. As described in the text, normal cells escape these outcomes, and are refractory to the mechanisms that trigger DNA damage and apoptosis in cancer cells.
