Fig. 2|. Intrahepatic drug targets in phase 2 and 3 clinical trials for NASH.
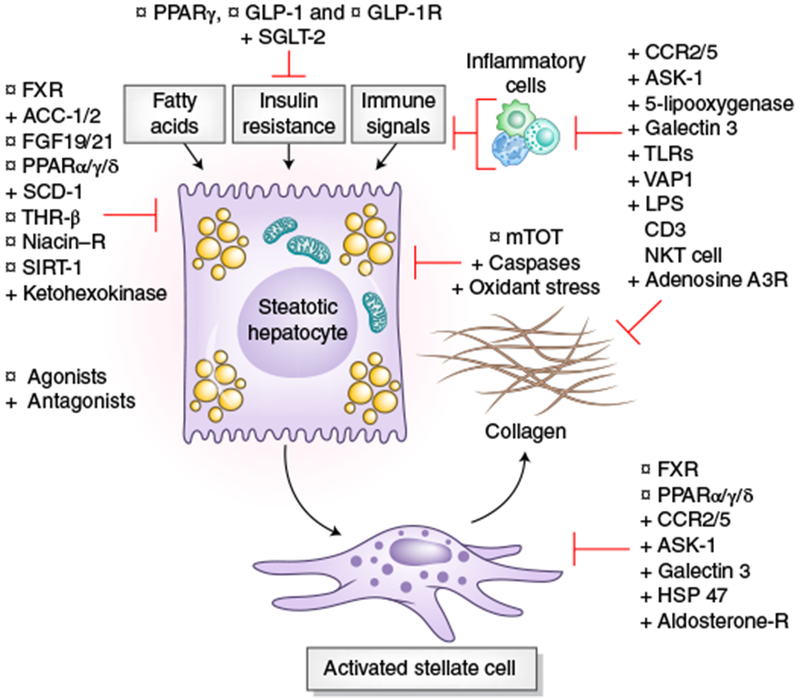
Depiction of the sites of action of drugs that are currently in phase 2 or 3 clinical trials, based on their primary locus of activity within the liver. Targets include those that regulate lipids and glucose homeostasis, and oxidant stress and mitochondrial targets in hepatocytes, inflammatory signals converge on hepatocytes, and those inflammatory signals and intracellular targets related to stellate cell activation and fibrogenesis. Gray boxes indicate disease drivers. Some targets (e.g, FXR agonists, CCR2 and CCR5 (CCR2/5) antagonist) have more than one action within the injury milieu. Agonists are indicated with a circle and antagonists with a cross. DGAT, Diacylglycerol O-acyltransferase; SCD, steroyl CoA-desaturase; THR, thyroid hormone receptor; SIRT, sirtuin; GLP, glucagon-like peptide; SGLT, sodium–glucose cotransporter; VAP, vascular adhesion protein; LPS, lipopolysaccharide; PPARα/δ/γ, peroxisome proliferator–activated receptors PPARα, PPARδ and PPARγ. Credit: Marina Corral Spence/Springer Nature
