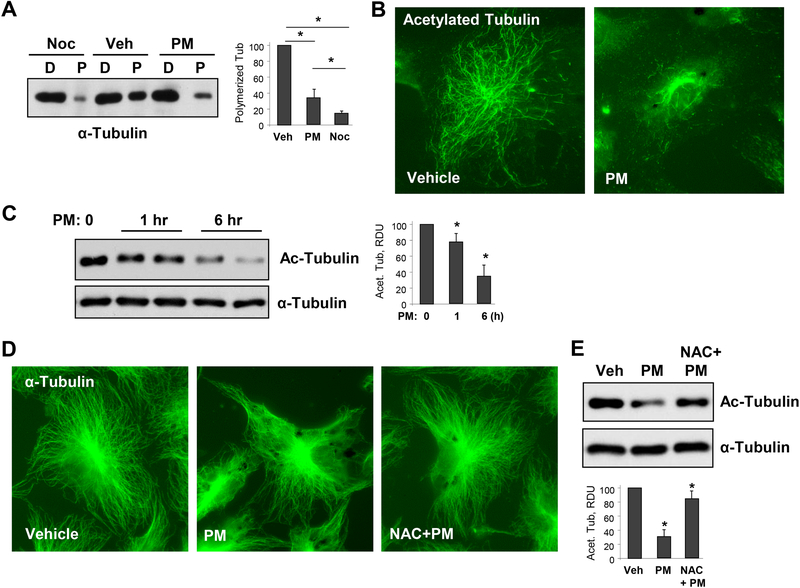
Figure 2. PM causes MT destabilization.
A - Cells were treated with vehicle, PM (20 μg/cm2 6 hrs), or MT destabilizing agent nocodazole (Noc, 0.5 μM, 30 min) and MT fractionation was performed as described in Methods. Equal total protein amounts from both soluble (depolymerized, D) and pellet (polymerized, P) cellular fractions were run for Western blotting to detect α-tubulin. Bar graphs depict quantitative densitometry analysis of polymerized α-tubulin bands in western blot experiments; n=5; **p<0.05. Data are expressed as mean + SD. B - HPAECs were treated with vehicle or PM (20 μg/cm2, 6 hrs). The cells were then subjected for immunofluorescence staining with antibody to acetylated tubulin. Results are representative of three independent experiments. C - Cells were treated with 20 μg/cm2 of PM for 1 hr or 6 hrs, and cell lysates were analyzed by Western blotting to detect acetylated α-tubulin. Probing for total α-tubulin was used as a normalization control. Bar graphs depict quantitative densitometry analysis of western blot experiments; n=3; **p<0.05. Data are expressed as mean + SD. D - HPAECs were pre-treated with vehicle or N-acetyl cysteine (NAC, 1mM, 30 min) followed by stimulation with vehicle or PM (20 μg/cm2, 6 hrs). The cells were then subjected for immunofluorescence staining with α-tubulin antibody. Results are representative of three independent experiments. E - Cells pretreated with vehicle or NAC (1mM, 30 min) were challenged with PM (20 μg/cm2, 6 hrs) and acetylated α-tubulin was analyzed by Western blotting with acetylated α-tubulin antibody. Probing for total α-tubulin was used as a normalization control. Bar graphs depict quantitative densitometry analysis of western blot experiments; n=3; **p<0.05. Data are expressed as mean + SD.