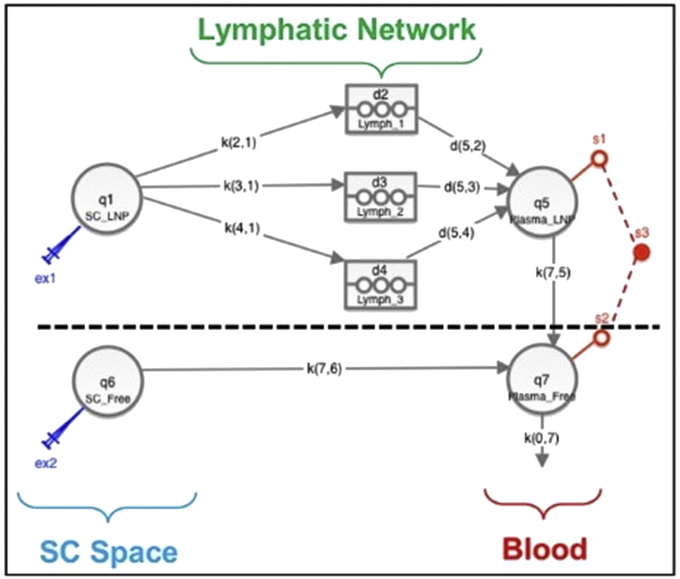
Figure 1.
MBPK model schematics for the pharmacokinetics of DcNP subcutaneously administered. The model has been thoroughly presented elsewhere.12 Briefly, the model features 2 portions, one for the DcNP uptake (above dashed line) and one for the free drug uptake (below dashed line). Above dashed line: Uptake of DcNPs from injection site (q1 – SC_LNP) to the lymphatics (k21, k31, k41) and subsequent release into the blood circulation (d52, d53, d54) was modeled by 3 successive, time-delayed compartments (d2 – Lymph_1, d3 – Lymph_2, d4 – Lymph_3). Liberation of free drug from DcNP (k75) was assumed to occur once the nanoparticles reaches the blood circulation (q5 – Plasma_LNP). Below dashed line: Uptake of free drugs from injection site (q6 – SC_Free) to blood circulation (q7 – Plasma_Free) was modeled by a free drug uptake rate (k76). To q7 – Plasma_Free connects q5 – Plasma_LNP. Plasma concentration measurements (s3) were the sum of systemic q7 – Plasma_Free and q5 – Plasma_LNP concentrations (s1, s2), being plasma concentrations of DcNP and equivalent free form measured indistinguishably. Parameters K represented linear kinetics rates. Based on drug-particle association data, the model assumed that, at the subcutaneous site, ATV and RTV were 100% incorporated in the DcNP, whereas TFV was only 10% associated (above dashed line), whereas the 90% fraction of the injected TFV dose was assumed to be free (below dashed line).