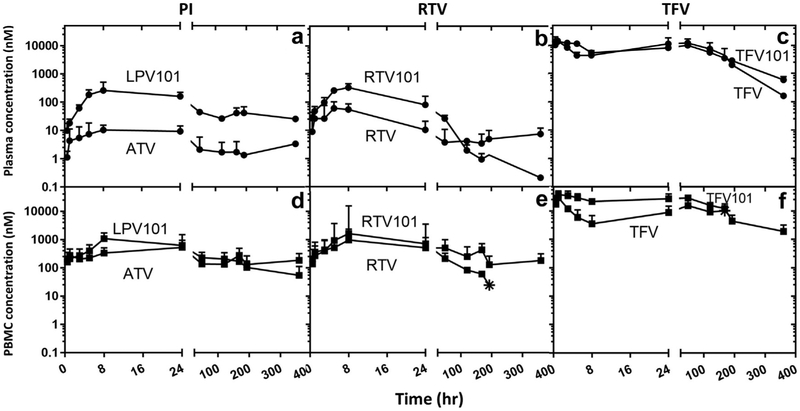
Figure 3.
Effects on plasma and blood mononuclear cells in nonhuman primates when substituting protease inhibitors, ATV in place of LPV. A single subcutaneous dose of ATV, RTV, and TFV drug was administered as nanocombination (DcNP) and compared with previously reported TLC-ART 101 LPV, RTV, and TFV.4 The 3 drug concentrations in plasma and PBMCs were monitored over 14 d (336 h). TLC-ART 101 drug plots are appended with “101” to distinguish them from current DcNP drugs. Upper panels (a-c) describe plasma concentration comparison of current ATV-RTV-TFV DcNP and previously reported TLC-ART 101 LPV-RTV-TFV. Lower panels (d-f) describe time-course of PBMC concentration comparison of current ATV-RTV-TFV DcNP and previously reported TLC-ART 101 LPV-RTV-TFV. Current ATV-RTV-TFV DcNP dosages were scaled to dosages equivalent to those in TLC-ART 101. Circle symbols are plasma timepoints. Square symbols are PBMC time-points. Star symbols represent the last detectable timepoint. Limit of detection 0.01-0.03 pmol/mL. No data points were plotted for measured drug levels that fell below the limit of detection. Data expressed were geometric mean (±SD).