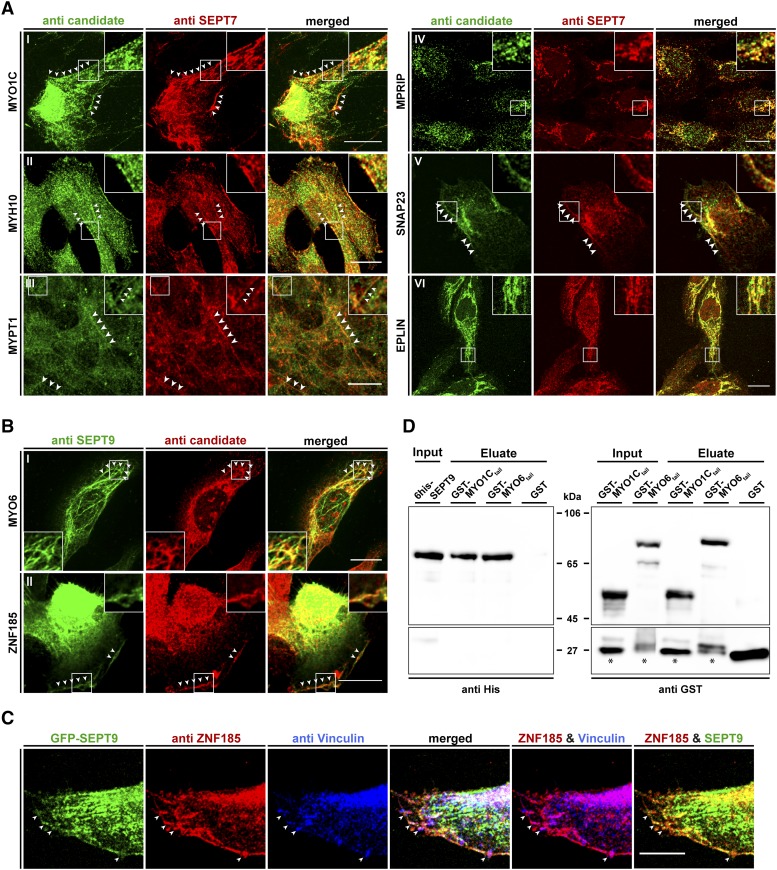
Figure 5.
Colocalization of interaction partners with the septin cytoskeleton in 1306 fibroblast cells and pulldown analysis showing a direct interaction of SEPT9 with myosin motors. A) The indicated candidate proteins were immunostained with a suitable primary antibody, followed by an Alexa488 coupled secondary antibody. The endogenous septin cytoskeleton was immunostained by an anti-SEPT7 primary antibody followed by an Alexa555 coupled secondary antibody. White arrowheads mark colocalizing structures. B) Colocalization of MYO6 and ZNF185 with the endogenous septin cytoskeleton by immunostaining of SEPT9 with an anti-SEPT9 primary antibody followed by an Atto488 coupled secondary antibody. Due to species incompatibility of the available primary antibodies against MYO6 and ZNF185, these two candidates had to be visualized via an Alexa555 coupled secondary antibody. White arrowheads mark colocalizing structures. C) Colocalization of ZNF185, Vinculin and SEPT9 in GFP-SEPT9 expressing cells. ZNF185 was visualized as in figure part B and Vinculin was visualized by an antibody directly coupled to Alexa647. White arrowheads mark sites of focal adhesions. Images in parts A-C were assembled from z-projections. The scale bars represent 20 µM. D) In vitro pulldown experiment showing direct binding of 6his-SEPT9 to tail fragments of myosin motors. GST-MYO6835-1294, GST-MYO1C809-1063 and free GST as control were immobilized on Glutathione sepharose and incubated with purified 6his-SEPT9. Samples of the SEPT9 input, the GST-fusion protein input and the eluates from the beads were subjected to SDS-PAGE and subsequent Western blot analysis using anti-his or anti-GST primary antibodies, respectively. Bands marked with an asterisk represent a GST containing degradation product of the GST-myosin fusion proteins.