Most of the methane produced on our planet gets naturally oxidized by a group of methanotrophic microorganisms before it reaches the atmosphere. These microorganisms are able to oxidize methane, both aerobically and anaerobically, and use it as their sole energy source. Although methanotrophs have been studied for more than a century, there are still many unknown and uncultivated groups prevalent in various ecosystems. This study focused on the diversity and adaptation of aerobic methane-oxidizing bacteria in different environments by comparing their phenotypic and genotypic properties. We used lab-scale microcosms to create a countergradient of oxygen and methane for preenrichment, followed by classical isolation techniques to obtain methane-oxidizing bacteria from a freshwater environment. This resulted in the discovery and isolation of a novel methanotroph with interesting physiological and genomic properties that could possibly make this bacterium able to cope with fluctuating environmental conditions.
KEYWORDS: methane, aerobic methane oxidation, comparative genomics, paddy field, soil microbiome
ABSTRACT
Methane-oxidizing microorganisms perform an important role in reducing emissions of the greenhouse gas methane to the atmosphere. To date, known bacterial methanotrophs belong to the Proteobacteria, Verrucomicrobia, and NC10 phyla. Within the Proteobacteria phylum, they can be divided into type Ia, type Ib, and type II methanotrophs. Type Ia and type II are well represented by isolates. Contrastingly, the vast majority of type Ib methanotrophs have not been able to be cultivated so far. Here, we compared the distributions of type Ib lineages in different environments. Whereas the cultivated type Ib methanotrophs (Methylococcus and Methylocaldum) are found in landfill and upland soils, lineages that are not represented by isolates are mostly dominant in freshwater environments, such as paddy fields and lake sediments. Thus, we observed a clear niche differentiation within type Ib methanotrophs. Our subsequent isolation attempts resulted in obtaining a pure culture of a novel type Ib methanotroph, tentatively named “Methylotetracoccus oryzae” C50C1. Strain C50C1 was further characterized to be an obligate methanotroph, containing C16:1ω9c as the major membrane phospholipid fatty acid, which has not been found in other methanotrophs. Genome analysis of strain C50C1 showed the presence of two pmoCAB operon copies and XoxF5-type methanol dehydrogenase in addition to MxaFI. The genome also contained genes involved in nitrogen and sulfur cycling, but it remains to be demonstrated if and how these help this type Ib methanotroph to adapt to fluctuating environmental conditions in freshwater ecosystems.
IMPORTANCE Most of the methane produced on our planet gets naturally oxidized by a group of methanotrophic microorganisms before it reaches the atmosphere. These microorganisms are able to oxidize methane, both aerobically and anaerobically, and use it as their sole energy source. Although methanotrophs have been studied for more than a century, there are still many unknown and uncultivated groups prevalent in various ecosystems. This study focused on the diversity and adaptation of aerobic methane-oxidizing bacteria in different environments by comparing their phenotypic and genotypic properties. We used lab-scale microcosms to create a countergradient of oxygen and methane for preenrichment, followed by classical isolation techniques to obtain methane-oxidizing bacteria from a freshwater environment. This resulted in the discovery and isolation of a novel methanotroph with interesting physiological and genomic properties that could possibly make this bacterium able to cope with fluctuating environmental conditions.
INTRODUCTION
Methanotrophs are a functional group of diverse Gram-negative bacteria that are defined by their ability to oxidize methane, which they utilize as a source of carbon and energy (1–3). Since their discovery in 1906 by Soehngen, they are known to play a key role in the global methane cycle through the reduction of methane emissions to the atmosphere (4–6). Aerobic methanotrophs utilize methane via a methane monooxygenase (MMO) that exists in a soluble (sMMO) cytoplasmic- and particulate (pMMO)-membrane-bound form, both of which catalyze the first step of methane oxidation to methanol (2). Methane-oxidizing bacteria (MOB) are ubiquitous in nature and have been found in various environments where oxygen and methane are readily available (1, 7). While most grow best with moderate pHs and temperature ranges, psychrophilic, thermophilic, alkaliphilic, and acidophilic methanotrophs have been isolated as well (reviewed in reference 2).
To date, the best-studied methanotrophs belong to the proteobacterial classes Alpha- and Gammaproteobacteria (2, 8), but MOB within the phyla Verrucomicrobia and NC10 (9–11) were recently discovered, expanding the phylogenetic diversity of MOB. Despite this diversity, MOB have remarkably similar methane oxidation pathways, while incorporating different pathways for carbon fixation. Proteobacterial MOB utilize C1 compounds via the ribulose monophosphate (RuMP) or serine pathways (3, 12), while verrucomicrobial MOB and NC10 bacteria use the Calvin cycle (13, 14). After the extensive isolation and characterization of methanotrophs that took place in the 1970s, three types of methanotrophs were defined (15, 16). The strains that incorporated carbon into biomass using the RuMP pathway contained intracytoplasmic membranes as vesicular disks, and monounsaturated hexadecenoic (16:1) signature fatty acids were grouped under type I. Type II strains differed from type I strains by utilizing the serine pathway for carbon fixation, having intracytoplasmic membranes aligned along the periphery of the cell and monounsaturated octadecenoic acid (18:1) as a major membrane lipid (12, 15).
In various studies, an additional group of methanotrophs has been described as type X (17, 18), defined originally based on genomic G+C content and intracytoplasmic membrane organization. This group had characteristics that did not define them under one type, possessing the full RuMP pathway as well as ribulose-1,5-bisphosphate carboxylase, indicative of the Calvin cycle, and at the time were considered to be adapted to higher temperatures. A combination of biochemical and molecular analyses, however, has revealed that type X strains should be reclassified under type I methanotrophs, and this clade is now referred to as type Ib (8). Nonetheless, these classifications do not encompass all isolates, with some having unexpected characteristics. For instance, a type II strain possessing signature membrane lipids that resemble type I methanotrophs (19) and Methylothermus thermalis, a gammaproteobacterium that possesses both 16:0 and 18:1 fatty acids typical for type I and II methanotrophs, respectively (20), have been reported.
Within the last 20 years, the genera containing MOB within the Proteobacteria have expanded to 23 (reference 21 and the references therein). With the exception of low-pH peat-adapted Methylocella (22) and Methyloferula (23), which possess only sMMO, all known methanotrophs encode a pMMO (24). The genes for pMMO (pmoCAB) but mainly pmoA, encoding pMMO subunit A, have been used to survey the MOB diversity in various ecosystems (25–27). These studies have shown remarkable environmental diversity, even within the comparably well-studied proteobacterial clades. Although within the Gammaproteobacteria there have been 12 genera of both type Ia and Ib that contain cultivars, isolates are lacking for the many uncultivated environmental sequence clusters (2).
Type Ib methanotrophs are known to possess a high metabolic diversity (28, 29). However, this diversity is still to be fully explored due to the many clades of environmental sequences lacking any isolate. These sequences cover a vast variety of natural habitats, such as peat, upland and wetland soil, hot springs, lakes, rivers, ground water, and the deep sea, potentially representing highly diverse metabolic capabilities (4, 30–32). The presence of multiple pathways for carbon and nitrogen fixation and assimilation and of both soluble and particulate MMOs makes it difficult to generalize when discussing physiological abilities of type Ib methanotrophs or any other type of MOB (33).
Methylococcus capsulatus is the only well-described type Ib organism, and it has since become the model organism for the entire group (34). However, sequences from this group are found mostly in upland soil (35). Presently, most known type Ib organisms seem to occur in freshwater environments, but only a few isolates have been described. These have a tendency to live very close to a methane source and under oxygen-limited conditions (36, 37). In this study, we isolated a novel type Ib methanotroph, tentatively named “Methylotetracoccus oryzae” strain C50C1, from a freshwater ecosystem and performed physiological and genomic characterization. Based on observations from electron microscopy and sequence analyses, it belongs to a novel genus that is widely distributed in paddy fields and lake ecosystems, making it a potential model representative for this group. We, furthermore, compared different physiological aspects of this isolate (habitat distribution, optimum growth temperature and pH, and key enzymatic activities) to those of other known isolates within the type Ib methanotrophs.
RESULTS AND DISCUSSION
Isolation of a gammaproteobacterial methanotroph from paddy soil.
Incubation of paddy field soil in a methane/oxygen countergradient microcosm and further purification of enriched bacteria on nitrate mineral salts (NMS) medium resulted in three gammaproteobacterial methanotrophs that were classified as type Ib. One strain (referred to as strain C50C1) was further purified via several transfers in liquid NMS medium until a pure culture was obtained.
Strain C50C1 was represented by Gram-negative and nonmotile cocci or coccoids (1.1 to 1.4 by 1.3 to 1.8 μm in size), which reproduced by binary fission and occurred singly, in pairs, or in tetrads or formed large cell clusters in old (≥2-week) cultures (Fig. 1A to C). Examination of thin-sectioned cells of strain C50C1 revealed a typical Gram-negative structure of the cell wall and the presence of intracytoplasmic membranes, arranged as stacks of vesicular disks (Fig. 1D), which is characteristic of type I methanotrophs. Globular structures apparently representing an S layer were observed on the cell surface (Fig. 1E). Although the presence of S layers is highly characteristic for many type I methanotrophs, including Methylococcus species (38), this type of S-layer symmetry has not been reported for any of the previously described methanotrophs.
FIG 1.
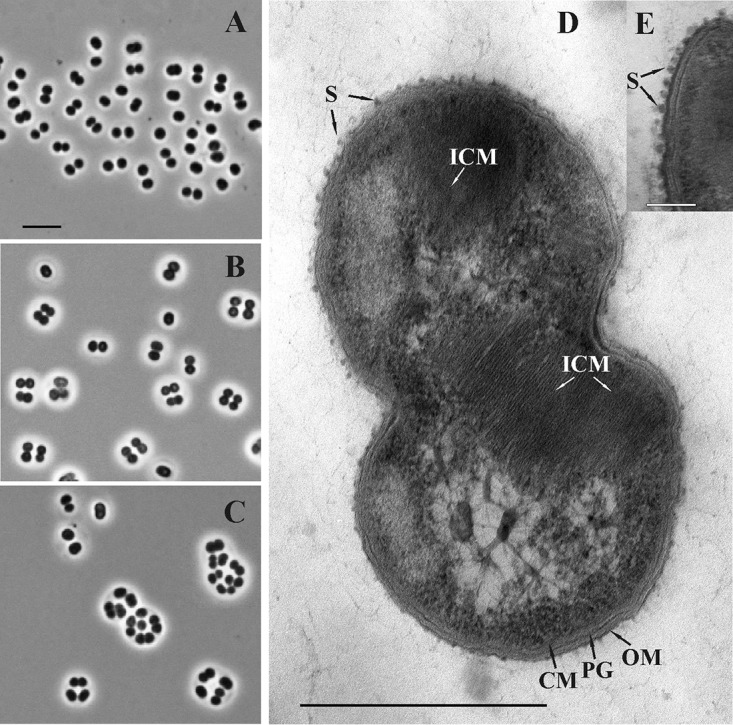
(A, B, C) Phase-contrast micrographs demonstrating the cell morphology of strain C50C1 in 4-, 7-, and 14-day-old cultures. Bar, 5 μm. (D, E) Electron micrograph of an ultrathin section of a cell. ICM, intracytoplasmic membranes; CM, cytoplasmic membrane; OM, outer membrane; PG, peptidoglycan layer; S, S layer. Bars, 0.5 μm (D) and 0.1 μm (E).
Strain C50C1 was able to grow only on methane and methanol. Methanol supported growth in the concentration range of 0.1 to 4% (vol/vol); the highest growth rates (doubling time, 21 h) occurred at 3% (vol/vol). No growth was observed on multicarbon compounds. Strain C50C1 grew in the pH range of 4.8 to 8.3, with the optimum at pH 6.8 to 7.5. The temperature range for growth was 4 to 30°C, with the optimum at 18 to 25°C. The doubling time on methane and methanol under optimal growth conditions was 16 and 21 h, respectively. Strain C50C1 was highly sensitive to salt stress and growth was inhibited at NaCl concentrations above 0.3% (wt/vol).
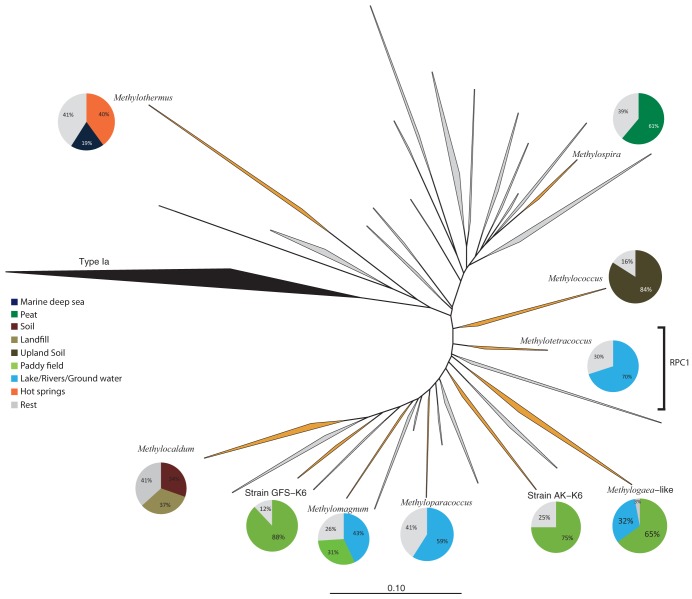
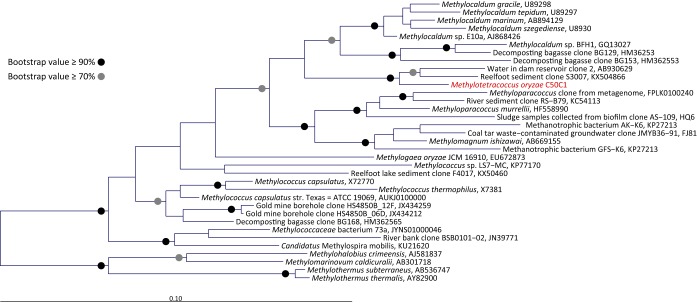
Based on 16S rRNA and pmoA gene-based phylogeny, strain C50C1 could be classified as type Ib methanotroph affiliated with rice paddy cluster 1 (RPC1) (Fig. 2). RPC1 forms a monophyletic lineage, containing pmoA sequences that were mostly retrieved from freshwater environments such as lakes, groundwater and paddy fields (25, 39, 40). So far, few members of type Ib methanotrophs have been characterized, resulting in the description of five genera. However, most clusters contain environmental sequences only and lack cultured representatives (Fig. 2). Closest cultivated relatives of strain C50C1 include Methylococcus capsulatus, Methylocaldum gracile and Methyloparacoccus murrellii (94% 16S rRNA gene identity to each species and 92% amino acid identity to the PmoA of M. capsulatus).
FIG 2.
Phylogenetic inference of methane monooxygenase (PmoA) protein sequences of type Ib methanotrophs. The tree is constructed using ARB’s neighbor-joining method. Type Ia sequences were used as the outgroup. Clades in orange are represented by isolates and clades in gray by environmental sequences only. All clusters that contain isolates are accompanied by a pie chart, with colors representing the environments to which the majority of sequences belong. RPC1, rice paddy cluster 1. The bar indicates 0.1 substitution per amino acid position.
Phenotype and growth characteristics of strain C50C1.
We made a phenotypic comparison between strain C50C1 and other type Ib isolates (Table 1). C50C1 grows on methane and methanol as sole energy sources (Table S2), is able to fix N2 (see Fig. S1 in the supplemental material), and grows at temperatures between 4 and 30°C, which is a much larger range than those of other characterized type Ib methanotrophs (Table 1). Similar to other MOB, it prefers pH values between 6 and 8 and is sensitive to 0.3% NaCl. Major phospholipid-derived fatty acids (PLFAs) in strain C50C1 are C16:1ω9c, C16:1ω7c, and C16:0. C16:1ω9c is highly unusual for type Ib methanotrophs, but small amounts have also been detected in Methylogaea and Methyloparacoccus (Table 1; Table S3). Large amounts of this PLFAs have so far been detected only in MOB belonging to Alphaproteobacteria (39), and its presence in strain C50C1 gives it a specific signature. The recently described Methyloterricola oryzae belonging to RPC1 possesses mainly C16:0, C16:1ω6c, and C16:1ω7c, typical of type Ib methanotrophs (41). Based on the complete PLFA profile, however, C50C1 is most closely related to Methyloterricola oryzae, strengthening its placement in RCP1 (Fig. S2). Furthermore, both PmoA (Fig. 2) and 16S rRNA gene-based phylogeny (Fig. 3) show a clear affiliation of strain C50C1 with the type Ib MOB.
TABLE 1.
Comparison of strain C50C1 growth characteristics to those of other type Ib methanotrophs
| Character- istic(s) |
Result fora
: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Methylo- tetracoccus oryzae C50C1 |
Methylo- paracoccus (2 strains) |
Methylo- coccus capsulatus (2 strains) |
Methylo- caldum (4 strains) |
Strain GFS-K6 |
Methyl- ogaea oryzae |
Methylo- magnum |
Strain AK-K6 |
Methylo- spira cluster |
|
| Isolation source, country(ies) |
Rice field, Italy |
Pond water, South Africa and Japan |
Thermal bath water, UK |
Marine sediment |
Terrestrial methane seep pond sediments, Bangladesh |
Rice field, Uruguay |
Rice fields, Bangladesh and Japan |
Warm spring sediments, Armenia |
Acidic sphagnum peat bog, Russia |
| PmoA cluster |
Freshwater sediment 2 (RPC1) |
Freshwater sediment 2 (RPCs) |
Methylo- coccus-like |
Methylo- caldum-like |
Methylo- coccaceae family |
JRP-4 |
Methylo- coccus- Methylo- caldum- Methylo- paracoccus- Methylo- gaea clade |
Methylo- coccaceae family |
OSC |
| Major habitat |
Freshwater lake |
Freshwater lake |
Meadow/ shrubs |
Soil | Rhizosphere/ root |
Paddy field |
Lake sediment/ soil |
Paddy field | Peat |
| Growth temp (°C) range, optimum |
4–30 | 20–37, 25–33 |
28–55, 37–50 |
20–62 | 8–35, 25–28 |
20–37, 30–35 |
20–37, 31–33 |
8–35, 25–28 |
8–25, 14–25 |
| pH range, optimum |
6–8 | 5.8–9, 6.3–6.8 |
5.5–9.0, ND |
5–9, 6–8 |
5.0–7.5, 6.4–7.0 |
5–8, 6.5–6.8 |
5.5–9.0, 6.8–7.4 |
5.0–7.5, 6.4–7.0 |
4.2–6.0, 6.0–6.5 |
| Tolerence to 1% NaCl |
No | No | Yes | ND | No | No | No | No | No |
| Key enzyme activities of sMMO, nitrogenase, RubisCO |
–, +, – |
–, –, – |
+, +, + |
–, –, + |
–, +, + |
–, –, – |
+, –, – |
–, +, + |
–, +, + |
| Cell morph- ology |
Cocci | Cocci | Cocci, rods |
Rods, pleomorphic |
Rods | Curved rods |
Rods | Rods | Curved rods (spiral) |
| Motility | None | None | Variable | Yes | None | None | Yes | None | Yes |
| Major fatty acid(s) |
C16:1ω9c, C16:1ω7c, C16:0 |
C16:1ω7c* | C16:0, C16:1ω7c* |
ND | C16:1ω7c | C16:0 | C14:0, C16:0, C16:1ω7c* |
C16:1ω7c | ND |
| Cell size (μm) |
1.1–1.4 by 1.3–1.8 |
0.8–1.5 | 0.8–1.5 by 1.0–1.5 |
0.6–1.2 by 1.0–1.8 |
1.5–2.2 0.5–1.5 |
0.5–0.7 by 2.0–2.2 |
1.5–2.0 by 2.0–4.0 |
1.5–2.2 by 0.5–1.5 |
1.0–1.5 by 2.0–2.5 |
| Pigmenta- tion |
White to brown |
White | White to brown |
Brown | White | White | White | White | ND |
| Forma- tion of: |
|||||||||
| Cysts | – | – | + | + | – | – | + | – | – |
| Chains | + | – | + | + | – | – | – | – | – |
| DNA G+C content (mol%) |
62.77 | 65.6 | 59–66 | 56.5–57.2 | ND | 63.1 | 64.1 | ND | ND |
| Reference(s) | Current study |
Hoefman et al. (80) |
Bowman et al. (8) |
Takeuchi et al., (43), Bodrossy et al. (42) |
Islam et al. (37) |
Geymonat et al. (81) |
Islam et al. (37), Khalifa et al. (47) |
Islam et al. (37) |
Danilova et al. (36) |
OSC, organic soil cluster; ND, not determined.
FIG 3.
16S rRNA gene-based phylogenetic analysis of a subgroup of closely related type Ib methanotrophs to strain C50C1 (in red), including isolates and environmental clones. Selected members of the Methylothermaceae were used to root the tree. The bar indicates 0.1 substitution per nucleotide position.
Growth dynamics of strain C50C1 in 5-fold-diluted ammonium-containing (circles) or nitrogen-free (squares) mineral salts medium (AMS and MS, respectively) under atmospheric (open symbols) and low (solid symbols) O2 levels. Optical density was measured at 600 nm. Arrows represent O2 replenishment. Download FIG S1, PDF file, 0.04 MB (335.5KB, pdf) .
Copyright © 2019 Ghashghavi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PLFA profiles of type I and verrucomicrobial MOB compared by nonmetric multiscale dimensional scaling (NMDS) of PLFA profiles of methanotroph cultures (expressed as percentages of total PLFA content). The two-dimensional distances between samples in the NMDS plot show the relative similarity between samples. The closest matching PLFA profiles are indicated with a minimum spanning tree analysis displaying the shortest distance (i.e., similarity) to connect all PLFA profiles, which results in relating a profile to its nearest neighbor for every sample. The analyses were carried out with the software PAST. Black dots, type Ia; blue squares, type Ib; red triangles, Methylothermaceae (type Ic); green dots, Methylacidiphilaceae (type III). Download FIG S2, PDF file, 0.3 MB (39KB, pdf) .
Copyright © 2019 Ghashghavi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Diversity and ecological niches of type Ib methanotrophs.
To gain an overview of the diversity and habitat preferences of cultivated and uncultivated type Ib methanotrophs, we performed a phylogenetic analysis of approximately 2,800 publicly available pmoA sequences from various environments. We classified the habitat information into eight environmental categories and compared the pmoA diversity to the environmental origins of the sequences (Fig. 2). Sequences could be grouped into 32 major sequence clusters. For a long time, only the genera Methylococcus and Methylocaldum were represented by isolates; however, recently several additional type Ib methanotrophs were obtained in pure culture (Fig. 2; Table 1 and the references within). Methylomagnum, Methylogaea, and strains SK-K6 and GFS-K6 all belong to clusters containing environmental sequences derived mainly from paddy fields. These isolates grow in similar pH ranges, but Methylogaea and Methylomagnum possess a slightly higher optimum growth temperature of 30 to 35°C.
Methyloparacoccus and the tentatively named Methylotetracoccus clades have most sequences derived from freshwater ecosystems. Since these strains have been isolated from similar environments, their growth parameters and genome-inferred physiological capabilities are highly similar. Contrastingly, both Methylococcus and Methylocaldum have been isolated from sources that differ from the major habitat of their respective sequence clade, based on environmental sequences. The former was isolated from a Roman thermal bath, the latter from marine sediment (7, 42, 43). Lastly, “Candidatus Methylospira mobilis” appears to be an accurate representative for its clade of mainly peat-derived environmental clones, as it is adapted to acidic conditions (36). Although type Ib MOB have shown to be diverse with regard to their environmental adaptability, they seem to play a very minor role in marine ecosystems, where most sequences belong to type Ia.
Genome sequencing of strain C50C1.
To gain further insights into the metabolic potential of strain C50C1, we sequenced and analyzed its genome. Assembly and binning resulted in a 4.83-Mbp draft genome consisting of 42 contigs longer than 1 kb. Based on single-copy marker gene analysis, the genome was predicted to be 99.1% complete, with 3.3% contamination. The overall G+C content is 63%. In total, the genome was predicted to contain 4,302 protein coding sequences (CDSs) and one copy of the rRNA operon. Genome size and G+C content are comparable to those of the four other sequenced type Ib methanotrophs, which range from 3.3 to 5 Mbp and 57% to 63%, respectively (Table 1). The rRNA operon copy numbers in bacterial genomes can vary from 1 to as many as 15 copies, and a correlation of copy number with resource availability has been hypothesized (44). Most other type Ib genomes also harbor only one copy, with the exception of Methylococcus capsulatus Bath, which contains two (34). Thus, MOB appear not to be in need of multiple rRNA copies for rapid adaptation to substrate availability, but this requires further analyses once more genomes of type Ib and other types of methanotrophs are sequenced.
Methane oxidation.
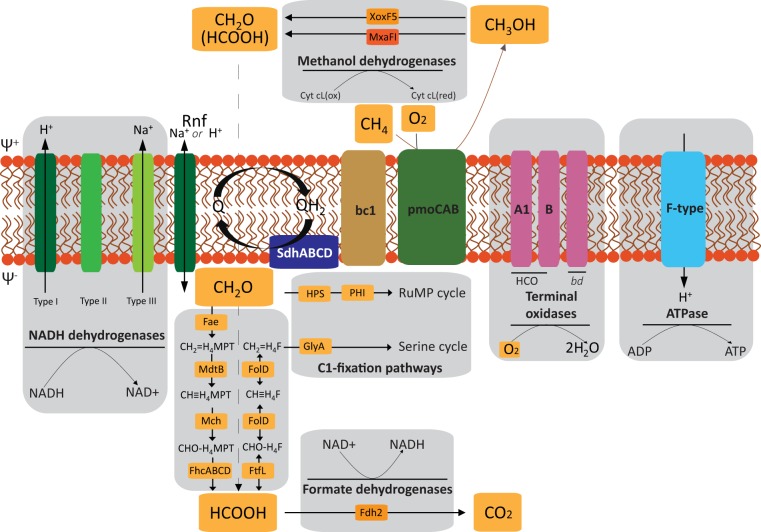
Based on the genomic information, the metabolic pathways for methane oxidation and energy conservation in strain C50C1 were reconstructed (Fig. 4). The genome includes two copies of the pmoCAB operon, encoding the membrane-bound pMMO, and four additional copies of pmoC, which are scattered throughout the genome. However, none of the two pmoCAB operons encodes the high-affinity pMMO-2 isoenzyme described in Alphaproteobacteria, which has been shown to be responsible for oxidation of methane at low mixing ratios (45). Since the concentrations of CH4 and O2 to which strain C50C1 would be exposed in its natural environment are not comparable to the ones experienced by atmospheric methane oxidizers, possessing a high-affinity pMMO would not necessarily be an advantage in a wetland. Neither the distinct pmoABC operon encoding the so-called pXMO (46) nor genes for the sMMO were identified in the genome, although the latter have been found in Methylococcus capsulatus (8) and in several Methylomagnum strains (37, 47) (Table 1 and references therein). According to recent studies, sMMO seems not to play a role in methanotrophy in paddy fields, as it was found to be absent in all rice field isolates, and PCR-based studies detected only mmoX genes related to Methylocystis/Methylosinus species (48).
FIG 4.
Predicted energy metabolism of strain C50C1. SdhABCD, succinate dehydrogenase; Rnf, NAD-ferredoxin reductase; Q, quinone; bc1, cytochrome bc1 complex; Fae, formaldehyde-activating enzyme; Mch, methenyl-H4MPT cyclohydrolase; FhcABCD, formyltransferase/hydrolase complex; FtfL, formate-tetrahydrofolate ligase; FolD, methylene-H4F dehydrogenase/cyclohydrolase; HPS, 3-hexulose-6-phosphate synthase; PHI, 6-phospho-3-hexuloisomerase; GlyA, serine hydroxymethyltransferase.
Methanol and formaldehyde oxidation.
For the subsequent oxidation of methanol to formaldehyde, the C50C1 genome encoded both the lanthanide-dependent XoxF5-type (49, 50) and the calcium-dependent MxaFI-type methanol dehydrogenase (MDH). The XoxF5-type MDH has been shown to have a higher affinity than MxaFI and, unlike the MxaFI-type enzyme, to directly convert methanol to formate in Methylacidiphilum fumariolicum SolV, which lacks a dedicated formaldehyde dehydrogenase (51, 52). However, XoxF-type enzymes were also shown to efficiently oxidize formaldehyde (53). In accordance with the dependency of XoxF-type MDHs on pyrroloquinoline quinone (PQQ), strain C50C1 also bears genes for PQQ biosynthesis. Electrons from the oxidation of methanol are transferred to cytochrome cL, which serves as the primary electron acceptor for MDH. In the periplasm, cytochrome cL is oxidized, and the electrons end up at typical membrane-bound terminal oxidases by way of class I c-type cytochromes (1).
Most of the reducing equivalents required for the metabolism of methane are produced by the oxidation of formaldehyde (3, 54). Formaldehyde is an important intermediate, as it forms the branching point for anabolic carbon fixation via the serine or RuMP cycle and catabolic substrate oxidation to CO2. However, this compound also is highly toxic, and its production and consumption consequently need to be tightly regulated (55).
A variety of enzymes have been shown to catalyze formaldehyde oxidation. Based on their electron acceptor, they can be grouped into nicotinamide adenine dinucleotide phosphate [NAD(P)+]-dependent and dye (cytochrome)-linked formaldehyde dehydrogenases (FalDH). Based on the genomic data, strain C50C1 possesses a homolog (74% amino acid identity) to a membrane-associated dye-linked PQQ-dependent FalDH putatively catalyzing formaldehyde oxidation. This enzyme in Methylococcus capsulatus Bath has been characterized (56) and was shown to be a member of the sulfide:quinone oxidoreductase enzyme family. Under high-copper growth conditions, this enzyme was found to be the major formaldehyde dehydrogenase. Additional homologs are present in Methylocaldum and Methylohalobius with, however, much lower identity (≤40%) and potentially different functions within the sulfide:quinone oxidoreductase family. C50C1 is lacking homologs of S-(hydroxymethyl) glutathione dehydrogenase (EC 1.1.1.284), which provides an alternative route from formaldehyde to formate in all other type Ib MOB.
Like other type Ib species, C50C1 has tetrahydrofolate (H4F) and 5,6,7,8-tetrahydromethanopterin (H4MPT)-linked C1 carrier pathways. H4MPT is the archaeal analogue of H4F and can transfer formyl, methenyl, methylene, and methyl groups (57). These two pathways were regarded as redundant. However, more recent observations have shown that formate might be a branching point for anabolic and catabolic reactions making these two pathways function in parallel (58). The generation of methylene H4F and its subsequent entry to the serine pathway is done through direct condensation of formaldehyde with H4F. Alternatively, methylene H4F can be formed from formate in the tetrahydromethanopterin pathway from H4MPT. The latter seems to occur in a facultative methylotrophic, non-methane-oxidizing Methylobacterium (59), thus making it likely to occur in strain C50C1 as well. In contrast to Methylobacterium, C50C1, furthermore, possesses FolD, a bifunctional methylene-H4F dehydrogenase and methenyl-H4F cyclohydrolase instead of the usual mtdA and fch gene pair, encoding enzymes catalyzing the separate reactions, respectively. In Methylobacterium chloromethanicum CM4, FolD has been shown to be specifically involved in dissimilation of the methyl-H4F (60). Although this process varies within MOB, all type Ib genomes analyzed to date with the exception of strain C50C1 encode the MtdA/Fch couple and lack FolD.
Formate oxidation.
In Methylococcus capsulatus Bath and Methylobacterium extorquens, two isoenzymes have been characterized to be involved in formate oxidation (61, 62). The first of these formate dehydrogenases (FDH-1) has been characterized as a tungsten-containing enzyme in M. extorquens and is arranged in a fdhABC gene cluster (61). While this enzyme has been identified in Methylococcus capsulatus Bath and M. capsulatus Texas, it is not present in other type Ib species, including strain C50C1. Contrastingly, the second FDH-2 is a molybdenum (Mo)-depending enzyme encoded by the fdhCBAD gene cluster. This enzyme is found in all other type Ib organisms, including strain C50C1, making it much more widespread than its tungsten-containing counterpart. In general, tungsten enzymes seem to be present mostly in anaerobic microbes, which may be a direct result of its availability and its higher redox properties than those of Mo in anoxic ecosystems (63). Functionally speaking, the two FDHs are virtually identical when their respective cofactor is present (61).
Energy conservation and respiration.
The draft genome of strain C50C1 encodes a complete electron transport chain, including a proton or sodium ion-translocating NAD-ferredoxin reductase (Rnf) complex, NADH:ubiquinone reductases (H+- and Na+-transporting types; complex I), succinate dehydrogenase (complex II), cytochrome bc1 complex enzymes (complex III), quinone-reducing cytochrome bd-type enzymes, and putatively cytochrome c-reducing heme-copper terminal oxidases (HCO; complex IV) and a FoF1-type ATPase (complex V) (Fig. 4).
The Rnf (Rhodobacter nitrogen fixation) complex is a novel ion-motive electron transport chain found in phylogenetically diverse prokaryotes. In Acetobacterium woodii, the Rnf complex catalyzes oxidation of Fdred, with concomitant reduction of NAD+ (64). The soluble B subunit (RnfB) of the complex is proposed to be the entry point for electrons from reduced ferredoxin. The C subunit (RnfC) mediates NADH reduction, thus serving as exit point of electrons. The free energy of this reaction is conserved in the electrogenic transport of protons or sodium ions across the membrane, thus establishing an electrochemical potential (64). The genomes of Methylobacter and Methylotenera encode this complex as well (65). Complex I transfers electrons from NADH into the quinone pool, which is coupled with the translocation of four protons across the inner membrane, further contributing to the formation of a proton motive force (pmf) that can be used to synthesize ATP by complex V. Complex II links the tricarboxylic acid (TCA) cycle to the respiratory chain by transferring the electrons derived from succinate oxidation into the quinone pool.
Previous studies have indicated that pMMO also is coupled to the electron transport chain at the level of quinone, with inhibitor studies providing additional evidence of this link (reference 56 and references therein). The oxidation of methane by the pMMO requires the additional activation by oxygen. As one oxygen atom of O2 is reduced to H2O and the second is incorporated into methane to form methanol, this results in a net consumption of two electrons per methane oxidized. Electrons from the subsequent oxidation of methanol or formaldehyde either end up in a membrane-bound class I c-type oxidase or directly enter into the quinone pool, respectively. The reduced quinol then transfers the electrons to the cytochrome bc1 complex, where the reduction of cytochrome c is linked to formation of pmf via the so-called Q-cycle. Complex IV finally uses the electrons obtained from cytochrome c to reduce O2 to H2O. This reaction is also linked to active translocation of protons, thus contributing to pmf.
The genome of strain C50C1 contains all of the subunits of two members of the HCO superfamily, encoding one A-family and one B-family terminal oxidase. B-family enzymes have been shown to be adapted to lower concentrations of oxygen than those of the A-family, resulting in a higher affinity for O2 but fewer protons pumped per electron (66). Possession of both A- and B-family HCO types may allow strain C50C1 to respire using a wide range of oxygen concentrations. This is further supported by the presence of a cytochrome bd oxidase, a respiratory quinol:O2 oxidoreductase with a very high O2 affinity (67). However, enzymes of the bd oxidase family conserve less energy than HCOs, as they derive electrons for O2 reduction directly from quinol and lack conserved channels for proton pumping, thus bypassing energy conservation at complexes III and IV (66, 67).
C1 fixation and nitrogen and sulfur metabolism.
Fixation of carbon and subsequent assimilation of formaldehyde occurs through the RuMP pathway in strain C50C1, which is typical for type Ib methanotrophs. Additionally, strain C50C1 encodes the serine cycle enzymes serine hydroxymethyl transferase (GlyA), phosphoenolpyruvate (PEP) carboxylase (Ppc), and malate dehydrogenase (Mdh). PEP carboxylase, which is a key enzyme of the serine cycle, is missing in both the Methylococcus and Methylocaldum genera. The PEP carboxylase encoded by C50C1 belongs to the nonregulated group of PEP carboxylases (68) whose activity is not controlled by intermediates of the TCA cycle or glycolysis/gluconeogenesis (69). Whether these additional enzymes give strain C50C1 an advantage over other type Ib enzymes remains to be investigated. Furthermore, all the enzymes for gluconeogenesis, the TCA cycle, and the nonoxidative pentose phosphate pathway are encoded in strain C50C1’s genome. Unlike with Methylocaldum marinum (43), Methylococcus capsulatus Bath (70), and strain GFS-K6 (37), ribulose-1,5-bisphosphate carboxylase/oxygenase is not encoded in the genome of strain C50C1 (Table 1).
A possible side reaction of the pMMO in MOB is the oxidation of ammonia to hydroxylamine (NH2OH). Subsequently, hydroxylamine is detoxified to produce nitrite and nitrous oxide (N2O), apparently without linking this reaction to energy conservation (71). Strain C50C1 possesses genes encoding cytochrome cd1 nitrite reductase (NIR), an NnrS protein involved in response to nitric oxide (NO), NO reductase (NOR), and lastly a NnrU family protein required for NIR and NOR expression. However, hydroxylamine oxidoreductase (HAO) or hydroxylamine reductase is missing from the genome of strain C50C1. As in other MOB, no chemolithotrophic growth was observed on ammonium in strain C50C1, and the apparent lack of hydroxylamine-detoxifying enzymes might contribute to an inability to cope with nitrogen stress caused by nitrification intermediates. However, it has been reported that M. denitrificans strain FJG1 under extreme hypoxia couples CH4 oxidation to nitrate reduction (72), which may be an explanation for the presence of denitrification genes in strain C50C1.
For nitrogen uptake and assimilation, strain C50C1 encodes three AmtB-type ammonium transporters, a NarK-type nitrate transporter, and assimilatory nitrate and nitrite reductases (encoded by napA and nirBD). Furthermore, the genome contained all genes for an active nitrogenase for growth under nitrogen-fixing conditions. These include two copies of the dinitrogenase subunits NifD and NifK, the dinitrogenase reductase NifH, as well as the Nif-specific regulatory protein NifA, two copies of the FeMo cofactor biosynthesis protein NifB, the cysteine desulfurase NifS, and the nitrogenase-stabilizing/protective protein NifW.
Like other methanotrophs, such as Methylosarcina lacus and Methylocaldum szegediense, strain C50C1 possesses the full soxYZ operon for sulfur oxidation along with the sulfite dehydrogenase SoxD and the sulfur oxidation molybdopterin protein SoxC. However, whether this genomic potential corresponds to an environmental relevance of strain C50C1 in the sulfur cycle remains to be investigated.
Description of Methylotetracoccus gen. nov.
Methylotetracoccus [Me.thy.lo.tet.ra.coc′cus]. N.L. n. methylum (from French me′thyle), the methyl group; N.L. pref. methylo, pertaining to the methyl radical; N.L. masc. subst. from Gr. adj. tetra, four; N.L. masc. n. coccus (from Gr. n. kokkos), a grain or berry; N.L. masc. n. Methylotetracoccus, referring to a methyl-using organism with tetrad-forming coccoid cells.
Gram-stain negative, nonmotile cocci or coccoids, which reproduce by binary fission and occur singly, in pairs, or in tetrads or form large cell clusters in old cultures. Cells contain intracytoplasmic membranes, arranged as stacks of vesicular disks. Strictly aerobic, neutrophilic, mesophilic, and nonthermotolerant. Members of the genus are obligate utilizers of C1 compounds, such as methane and methanol. Methane is oxidized by pMMO, with sMMO and pXMO being absent. Cells are capable of dinitrogen fixation. The major PLFAs are C16:1ω9c, C16:1ω7c, and C16:0. The most closely related genera are Methyloparacoccus, Methylocaldum, and Methylomagnum within the family Methylococcaceae in the class Gammaproteobacteria. Known habitats are freshwater ecosystems, such as paddy fields and lake sediments.
Description of Methylotetracoccus oryzae sp. nov.
Methylotetracoccus oryzae (O′ryzae N.L. masc. adj. oryzae, pertaining to a paddy field).
Description is as for the genus, with the following amendments. Cells are 1.1 to 1.4 μm wide and 1.3 to 1.8 μm long. Growth occurs only on methane and methanol. Methanol supports growth in the range of concentrations of 0.1 to 4% (vol/vol); the highest growth rates with specific generation times of 0.033 h−1 (doubling time, 21 h) are observed at 3% (vol/vol). Optimal growth occurs at 18 to 25°C and pH 6.8 to 7.5. Highly sensitive to salt stress; growth is inhibited at NaCl concentrations above 0.3% (wt/vol). The type strain C50C1 was isolated from a paddy field in Cixi, Zhejiang Province, China. The G+C content of the type strain is 63 mol% (genome sequence).
Conclusions.
In this study, we isolated a novel type Ib methanotroph that can serve as a representative organism for the type Ib freshwater lineage. We report the high-quality draft genome of strain C50C1, which can help design further research to study the role of these MOB in the environment. Based on growth experiments along with genomic data, C50C1 seems to be an obligate methanotroph able to fix nitrogen. The draft genome indicates a potential for metabolic flexibility, with genetic modularity, including multiple methanol dehydrogenases, several pathways for formaldehyde oxidation, all enzymes of one and several enzymes of another pathway for C1 fixation, and several terminal oxidases. These genomic potentials may allow strain C50C1 to adapt to various environmental conditions, as already seen in its growth temperature range. The potential for sulfur oxidation within strain C50C1 and its environmental relevance need to be further investigated.
MATERIALS AND METHODS
Enrichment conditions and isolation approach.
Enrichments of methane-oxidizing bacteria were started from a paddy field soil sample in Cixi, Zhejiang Province, China (N30°11.066′; E121°21.351′). Soil characteristics and the sampling procedure are described in detail elsewhere (73). Preenrichment was carried out for 14 days in gradient microcosms supplied with 15% methane from the bottom compartment and ambient air from the top (74). After preincubation, the soil was harvested, diluted in nitrate mineral salts (NMS) medium (17) (see Table S1 in the supplemental material), and plated onto solid NMS medium containing 2% agarose. Plates were incubated in air-tight jars supplemented with ambient air and 20% methane. Selected colonies were streaked onto fresh plates to obtain single colonies. The latter, however, were composed not only of methanotrophic bacteria but also of satellite heterotrophic microorganisms. Selected colonies that contained the lowest number of satellite cells were picked and used to inoculate 30-ml serum vials containing 10 ml of 2-fold-diluted NMS medium together with 20 μl of trace element solution I and solution II (Table S1). After inoculation, the vials were sealed with rubber septa, and methane was added aseptically to attain a final mixing ratio of approximately 20% (vol/vol). The inoculated vials were then incubated at 24°C and 100 rpm. The cultures were examined by phase-contrast microscopy, and if morphologically uniform, the cells were transferred to fresh medium and grown again under the same growth conditions. This process of serial dilutions was repeated over 6 months until the target isolate, designated strain C50C1, was obtained in a pure culture. Once isolated, this methanotroph was maintained in 2-fold-diluted NMS medium and was subcultured at 4-week intervals.
Compositions of AMS, NMS, and MS media (as described by Whittenbury et al. [16]) and trace element solutions. Download Table S1, DOCX file, 0.02 MB (15.7KB, docx) .
Copyright © 2019 Ghashghavi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Substrate utilization pattern of strain C50C1. Download Table S2, DOCX file, 0.02 MB (15.2KB, docx) .
Copyright © 2019 Ghashghavi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phospholipid fatty acid (PLFA) profiles of strain C50C1. Download Table S3, DOCX file, 0.02 MB (16.5KB, docx) .
Copyright © 2019 Ghashghavi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phase-contrast and electron microscopy.
Morphological observations and cell size measurements were made with a Zeiss Axioplan 2 microscope and AxioVision 4.2 software (Zeiss, Jena, Germany). Cell morphology was examined by using batch cultures grown to the early-exponential, late-exponential, and stationary growth phases. For preparation of ultrathin sections, cells of the exponentially growing culture of strain C50C1 were collected by centrifugation and prefixed with 1.5% (wt/vol) glutaraldehyde in 0.05 M cacodylate buffer (pH 6.5) for 1 h at 4°C and then fixed with 1% (wt/vol) OsO4 in the same buffer for 4 h at 20°C. After dehydration in an ethanol series, the samples were embedded into Epon 812 epoxy resin. Thin sections were cut on an LKB-4800 microtome (LKB-Produkter AB, Stockholm, Sweden) and stained with 3% (wt/vol) uranyl acetate in 70% (vol/vol) ethanol. The specimen samples were examined with a JEM-100B transmission electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 80 kV.
Growth experiments.
Physiological tests were performed in liquid, 2-fold-diluted NMS medium with methane. The growth of strain C50C1 was monitored by measuring its optical density at 600 nm (OD600) for 2 weeks under a variety of conditions, including temperatures of 2 to 37°C, pHs of 4.0 to 8.5, and NaCl concentrations of 0 to 4.0% (wt/vol). Variations in pH were achieved by mixing 0.1 M solutions of H3PO4, KH2PO4, K2HPO4, and K3PO4. The utilization of potential carbon sources was examined using 0.05% (wt/vol) concentrations of the following compounds: formate, glucose, sucrose, galactose, lactose, fructose, citrate, succinate, pyruvate, acetate, and ethanol. The ability to grow on methanol was tested in NMS medium containing 0.01 to 5% (vol/vol) methanol.
Nitrogen fixation activity was assessed by monitoring growth in nitrogen-free medium. Incubations were performed in batches in triplicates. Bottles of 120 ml were sterilized and aseptically supplied with 17 ml of liquid 5-fold-diluted sterilized ammonium mineral salts (AMS) medium or 5-fold-diluted nitrogen-free mineral salts (MS) medium (Table S1). The headspace contained either an ambient or low O2 atmosphere (2%, vol/vol). Low O2 concentrations in the headspace were achieved by 5 rounds of vacuum application to the bottles, followed by flushing with N2-CO2 (90%/10%, vol/vol). Subsequently, 2% (vol/vol) O2 was added aseptically. All bottles received 10% (vol/vol) CH4 aseptically. Prior to inoculation, biomasses from 3 batch incubations pregrown on 5-fold-diluted AMS, NMS, or MS medium to mid-exponential phase were pooled. Cells were washed twice to remove any remaining nitrogen source by pelleting the biomass in 50-ml tubes at 1,000 × g for 10 min (5810 centrifuge; Eppendorf, Hamburg, Germany). Subsequently, the supernatant was removed and replaced with nitrogen-free, 5-fold-diluted MS medium. Cells were dissolved in 5-fold-diluted MS medium. All bottles were inoculated with 3 ml of the washed cells at a starting OD600 of 0.05. The OD600 was measured using a spectrophotometer (Spectronic200; ThermoFisher Scientific, Waltham, MA, USA). The CH4 concentrations in the headspace were measured by injection of 50-μl gas samples into an HP 5890 gas chromatograph (Hewlett Packard, Palo Alto, CA, USA) equipped with a Porapak Q 100/120 mesh (Sigma-Aldrich, Saint Louis, MO, USA) and a flame ionization detector (FID). O2 concentrations were determined using an Agilent 6890 series gas chromatograph coupled to a mass spectrometer (Agilent, Santa Clara, USA) equipped with a Porapak Q column heated at 80°C, with helium as the carrier gas, as described previously (75).
Molecular analyses.
Extraction, analysis, and identification of phospholipid-derived fatty acids (PLFAs), including dimethyl disulfide (DMDS) derivatization to determine double-bond positions, was performed as described by Dedysh et al. (19). DNA was extracted from 2 ml liquid culture using the PowerSoil DNA isolation kit (MO Bio Laboratories Inc., Carlsbad, CA, USA) according to the manufacturer’s protocol. The genomic DNA was sequenced on the Illumina MiSeq platform, with MiSeq reagent kit v3 (600 cycles, yielding 2× 300-bp paired-end sequencing reads; Life Technologies, Carlsbad, CA, USA). For genomic library preparation using the Nextera XT kit (Illumina, San Diego, CA, USA), in total 5 μl genomic DNA (gDNA) normalized to 0.2 ng/μl was used. Fragmentation was performed enzymatically, followed by incorporation of the indexing adapters and amplification of the library as described by the manufacturer. Purification of the amplified library was performed using AMPure XP beads, and the quality and size distribution of the library were checked using the Agilent 2100 Bioanalyzer and the high-sensitivity DNA kit (Agilent Technologies, Santa Clara, CA, USA). Fluorimetric quantitation of the library was performed by Qubit using the double-stranded DNA (dsDNA) HS assay kit (Thermo Fisher Scientific Inc., Waltham, USA). For normalization of the library, the concentration measured by Qubit and the average fragment size obtained with the Agilent 2100 bioanalyzer were used. After dilution to a 4 nM end concentration, the library was denatured and diluted according to the MiSeq System Denature and Dilute Libraries Guide (76) and loaded in the cartridge, and the sequence run was started using the Illumina MiSeq platform (Illumina, San Diego, CA, USA).
Bioinformatic analysis.
Illumina raw sequencing reads were imported into CLC Genomics Workbench (v11.0.2; Qiagen/CLCbio, Aarhus, Denmark) and trimmed on the bases of quality and length (≥100 bp), resulting in nearly 11.5 million reads, which were used for subsequent analyses. Reads were assembled using CLC Genomics Workbench (assembly parameters were a word size of 20, a bubble size of 50, and a minimum contig length of 200; mapping parameters were a mismatch cost of 2, an insertion cost of 3, a deletion cost of 3, a length fraction of 0.5, and a similarity fraction of 0.8). As a slight contamination in the culture used for DNA extraction was observed, metagenomic binning was performed based on C+G content and sequencing depth (77). The assembled genome of strain C50C1 was composed of 42 contigs with an N50 of 199.476 bp, an overall genome size of 4.8 Mbp, and an average G+C content of 63%. Genome completeness and contamination were estimated by CheckM (78) to be 99.1% and 3.3%, respectively. Binned contig sequences were submitted to the RAST automated annotation pipeline (79), which includes genomic object prediction (CDSs and RNA genes), sequence homology searches, prediction of protein localization, and reconstruction of metabolic networks. Subsequently, the annotation was refined manually and compared to publicly available genomes of aerobic MOB.
Data availability.
The high-quality draft genome of strain C50C1 is available at NCBI under BioProject accession number PRJNA361434.
ACKNOWLEDGMENTS
We thank Natalia E. Suzina for electron microscopy analyses and Huub Op den Camp for help with methanol dehydrogenase classification. Soil sampling was possible thanks to Zhihong.
This project was funded by the European Research Council (ERC advanced grant Ecomom 339880), the Netherlands Organisation for Scientific Research (NWO grants 863.14.019 and 016.Vidi.189.050), and BE-Basic (grant fp07.002.01). The initial enrichment and isolation were done as part of the Biogeochemistry of Paddy Soil Evolution framework, supported by the Deutsche Forschungsgemeinschaft (DFG) and the European Science Foundation EUROCORES program EuroEEFG. S.E.B. and S.N.D. acknowledge budgetary support (research topic 0104-2018-0034).
REFERENCES
- 1.Hanson RS, Hanson TE. 1996. Methanotrophic bacteria. Microbiol Rev 60:439–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semrau JD, DiSpirito AA, Yoon S. 2010. Methanotrophs and copper. FEMS Microbiol Rev 34:496–531. doi: 10.1111/j.1574-6976.2010.00212.x. [DOI] [PubMed] [Google Scholar]
- 3.Trotsenko YA, Murrell JC. 2008. Metabolic aspects of aerobic obligate methanotrophy. Adv Appl Microbiol 63:183–229. doi: 10.1016/S0065-2164(07)00005-6. [DOI] [PubMed] [Google Scholar]
- 4.Chistoserdova L. 2015. Methylotrophs in natural habitats: current insights through metagenomics. Appl Microbiol Biotechnol 99:5763–5779. doi: 10.1007/s00253-015-6713-z. [DOI] [PubMed] [Google Scholar]
- 5.Conrad R. 2009. The global methane cycle: recent advances in understanding the microbial processes involved. Environ Microbiol Rep 1:285–292. doi: 10.1111/j.1758-2229.2009.00038.x. [DOI] [PubMed] [Google Scholar]
- 6.Söhngen NL. 1906. Über bakterien, welche methan als kohlenstoffnahrung und energiequelle gebrauchen. Zentrabl Bakteriol Parasitenk Infektionskr 15:513–517. [Google Scholar]
- 7.Bowman J. 2006. The methanotrophs—the families Methylococcaceae and Methylocystaceae, p 266–289. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H (ed), The Prokaryotes, 3rd ed Springer, New York, NY. [Google Scholar]
- 8.Bowman JP, Sly LI, Nichols PD, Hayward A. 1993. Revised taxonomy of the methanotrophs: description of Methylobacter gen. nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes only the group I methanotrophs. Int J Syst Evol Microbiol 43:735–753. doi: 10.1099/00207713-43-4-735. [DOI] [Google Scholar]
- 9.Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MMM, Schreiber F, Dutilh BE, Zedelius J, de Beer D, Gloerich J, Wessels HJCT, van Alen T, Luesken F, Wu ML, van de Pas-Schoonen KT, Op den Camp HJM, Janssen-Megens EM, Francoijs K-J, Stunnenberg H, Weissenbach J, Jetten MSM, Strous M. 2010. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543. doi: 10.1038/nature08883. [DOI] [PubMed] [Google Scholar]
- 10.Op den Camp HJ, Islam T, Stott MB, Harhangi HR, Hynes A, Schouten S, Jetten MS, Birkeland NK, Pol A, Dunfield PF. 2009. Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environ Microbiol Rep 1:293–306. doi: 10.1111/j.1758-2229.2009.00022.x. [DOI] [PubMed] [Google Scholar]
- 11.Sangwan P, Kovac S, Davis KE, Sait M, Janssen PH. 2005. Detection and cultivation of soil Verrucomicrobia. Appl Environ Microbiol 71:8402–8410. doi: 10.1128/AEM.71.12.8402-8410.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whittenbury R, Colby J, Dalton H, Reed H. 1976. Biology and ecology of methane oxidizers, p 281–292. In Schlegel HC, Gottschalk G, Pfennig N (ed), Symposium on microbial production and utilization of gases (H2, CH4, CO), Göttingen. Akademie der Wissenschaften, Göttingen, Germany. [Google Scholar]
- 13.Khadem AF, Pol A, Wieczorek A, Mohammadi SS, Francoijs KJ, Stunnenberg HG, Jetten MS, Op den Camp HJ. 2011. Autotrophic methanotrophy in verrucomicrobia: Methylacidiphilum fumariolicum SolV uses the Calvin-Benson-Bassham cycle for carbon dioxide fixation. J Bacteriol 193:4438–4446. doi: 10.1128/JB.00407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasigraf O, Vogt C, Richnow HH, Jetten MSM, Ettwig KF. 2012. Carbon and hydrogen isotope fractionation during nitrite-dependent anaerobic methane oxidation by Methylomirabilis oxyfera. Geochim Cosmochim Acta 89:256–264. doi: 10.1016/j.gca.2012.04.054. [DOI] [Google Scholar]
- 15.Trotsenko YA. 1976. Isolation and characterization of obligate methanotrophic bacteria, p 329–336. In Schlegel HC, Gottschalk G, Pfennig N (ed), Symposium on microbial production and utilization of gases (H2, CH4, CO), Göttingen. Akademie der Wissenschaften, Göttingen, Germany. [Google Scholar]
- 16.Whittenbury R, Phillips K, Wilkinson J. 1970. Enrichment, isolation and some properties of methane-utilizing bacteria. Microbiology 61:205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 17.Whittenbury R. 1981. The interrelationship of autotrophy and methylotrophy as seen in Methylococcus capsulatus (Bath), p 181–190. In Dalton H. (ed), Microbial growth on C1 compounds. Heyden, London, United Kingdom. [Google Scholar]
- 18.Whittenbury R, Dalton H. 1981. The methylotrophic bacteria, p 894–902. In Starr MP, Stolp H, Trüper HG, Balows A, Schlegel EG (ed), The prokaryotes. Springer, Berlin, Germany. [Google Scholar]
- 19.Dedysh SN, Belova SE, Bodelier PL, Smirnova KV, Khmelenina VN, Chidthaisong A, Trotsenko YA, Liesack W, Dunfield PF. 2007. Methylocystis heyeri sp. nov., a novel type II methanotrophic bacterium possessing ‘signature’ fatty acids of type I methanotrophs. Int J Syst Evol Microbiol 57:472–479. doi: 10.1099/ijs.0.64623-0. [DOI] [PubMed] [Google Scholar]
- 20.Tsubota J, Eshinimaev BT, Khmelenina VN, Trotsenko YA. 2005. Methylothermus thermalis gen. nov., sp. nov., a novel moderately thermophilic obligate methanotroph from a hot spring in Japan. Int J Syst Evol Microbiol 55:1877–1884. doi: 10.1099/ijs.0.63691-0. [DOI] [PubMed] [Google Scholar]
- 21.Dedysh SN, Knief C. 2018. Diversity and phylogeny of described aerobic methanotrophs, p 17–42. In Kalyuzhnaya MG, Xing X-H (ed), Methane biocatalysis: paving the way to sustainability. Springer, Cham, Germany. [Google Scholar]
- 22.Dedysh SN, Liesack W, Khmelenina VN, Suzina NE, Trotsenko YA, Semrau JD, Bares AM, Panikov NS, Tiedje JM. 2000. Methylocella palustris gen. nov., sp. nov., a new methane-oxidizing acidophilic bacterium from peat bogs, representing a novel subtype of serine-pathway methanotrophs. Int J Syst Evol Microbiol 50:955–969. doi: 10.1099/00207713-50-3-955. [DOI] [PubMed] [Google Scholar]
- 23.Vorobev AV, Baani M, Doronina NV, Brady AL, Liesack W, Dunfield PF, Dedysh SN. 2011. Methyloferula stellata gen. nov., sp. nov., an acidophilic, obligately methanotrophic bacterium that possesses only a soluble methane monooxygenase. Int J Syst Evol Microbiol 61:2456–2463. doi: 10.1099/ijs.0.028118-0. [DOI] [PubMed] [Google Scholar]
- 24.McDonald IR, Murrell JC. 1997. The particulate methane monooxygenase gene pmoA and its use as a functional gene probe for methanotrophs. FEMS Microbiol Lett 156:205–210. doi: 10.1111/j.1574-6968.1997.tb12728.x. [DOI] [PubMed] [Google Scholar]
- 25.Knief C. 2015. Diversity and habitat preferences of cultivated and uncultivated aerobic methanotrophic bacteria evaluated based on pmoA as molecular marker. Front Microbiol 6:1346. doi: 10.3389/fmicb.2015.01346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengupta A, Dick WA. 2017. Methanotrophic bacterial diversity in two diverse soils under varying land-use practices as determined by high-throughput sequencing of the pmoA gene. Appl Soil Ecol 119:35–45. doi: 10.1016/j.apsoil.2017.05.031. [DOI] [Google Scholar]
- 27.Ghashghavi MS, Jetten MSM, Lüke C. 2017. Survey of methanotrophic diversity in various ecosystems by degenerate methane monooxygenase gene primers. AMB Express 7:162. doi: 10.1186/s13568-017-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madigan MT, Martinko JM, Parker J. 2017. Brock Biology of Microorganisms, 13th ed Pearson Benjamin Cummings, San Francisco, CA. [Google Scholar]
- 29.Wise MG, McArthur JV, Shimkets LJ. 1999. Methanotroph diversity in landfill soil: isolation of novel type I and type II methanotrophs whose presence was suggested by culture-independent 16S ribosomal DNA analysis. Appl Environ Microbiol 65:4887–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanson R, Wattenburg E. 1991. Ecology of methane-oxidizing bacteria, p 325–349. In Goldberg I, Rokem JS (ed), Biology of methylotrophs. Butterworths Publishers, London, United Kingdom. [Google Scholar]
- 31.Kip N, Van Winden JF, Pan Y, Bodrossy L, Reichart G-J, Smolders AJ, Jetten MSM, Damsté JSS, Op den Camp HJM. 2010. Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nature Geosci 3:617. doi: 10.1038/ngeo939. [DOI] [Google Scholar]
- 32.Murrell JC. 2010. The aerobic methane oxidizing bacteria (methanotrophs), p 1953–1966. In Timmis KN. (ed), Handbook of hydrocarbon and lipid microbiology. Springer, Heidelberg, Germany. [Google Scholar]
- 33.Roslev P, Iversen N, Henriksen K. 1997. Oxidation and assimilation of atmospheric methane by soil methane oxidizers. Appl Environ Microbiol 63:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward N, Larsen Ø, Sakwa J, Bruseth L, Khouri H, Durkin AS, Dimitrov G, Jiang L, Scanlan D, Kang KH, Lewis M, Nelson KE, Methé B, Wu M, Heidelberg JF, Paulsen IT, Fouts D, Ravel J, Tettelin H, Ren Q, Read T, DeBoy RT, Seshadri R, Salzberg SL, Jensen HB, Birkeland NK, Nelson WC, Dodson RJ, Grindhaug SH, Holt I, Eidhammer I, Jonasen I, Vanaken S, Utterback T, Feldblyum TV, Fraser CM, Lillehaug JR, Eisen JA. 2004. Genomic insights into methanotrophy: the complete genome sequence of Methylococcus capsulatus (Bath). PLoS Biol 2:e303. doi: 10.1371/journal.pbio.0020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knief C, Lipski A, Dunfield PF. 2003. Diversity and activity of methanotrophic bacteria in different upland soils. Appl Environ Microbiol 69:6703–6714. doi: 10.1128/aem.69.11.6703-6714.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danilova OV, Suzina NE, Van De Kamp J, Svenning MM, Bodrossy L, Dedysh SN. 2016. A new cell morphotype among methane oxidizers: a spiral-shaped obligately microaerophilic methanotroph from northern low-oxygen environments. ISME J 10:2734. doi: 10.1038/ismej.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Islam T, Larsen Ø, Torsvik V, Øvreås L, Panosyan H, Murrell JC, Birkeland N-K, Bodrossy L. 2015. Novel methanotrophs of the family Methylococcaceae from different geographical regions and habitats. Microorganisms 3:484–499. doi: 10.3390/microorganisms3030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khmelenina V, Suzina N, Trotsenko YA. 2013. Surface layers of methanotrophic bacteria. Microbiology 82:529–541. doi: 10.1134/S0026261713050068. [DOI] [PubMed] [Google Scholar]
- 39.Lüke C, Frenzel P. 2011. Potential of pmoA amplicon pyrosequencing for methanotroph diversity studies. Appl Environ Microbiol 77:6305–6309. doi: 10.1128/AEM.05355-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lüke C, Krause S, Cavigiolo S, Greppi D, Lupotto E, Frenzel P. 2010. Biogeography of wetland rice methanotrophs. Environ Microbiol 12:862–872. doi: 10.1111/j.1462-2920.2009.02131.x. [DOI] [PubMed] [Google Scholar]
- 41.Frindte K, Maarastawi SA, Lipski A, Hamacher J, Knief C. 2017. Characterization of the first rice paddy cluster I isolate, Methyloterricola oryzae gen. nov., sp. nov. and amended description of Methylomagnum ishizawai. Int J Syst Evol Microbiol 67:4507–4514. doi: 10.1099/ijsem.0.002319. [DOI] [PubMed] [Google Scholar]
- 42.Bodrossy L, Holmes EM, Holmes AJ, Kovács KL, Murrell JC. 1997. Analysis of 16S rRNA and methane monooxygenase gene sequences reveals a novel group of thermotolerant and thermophilic methanotrophs, Methylocaldum gen. nov. Arch Microbiol 168:493–503. doi: 10.1007/s002030050527. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi M, Kamagata Y, Oshima K, Hanada S, Tamaki H, Marumo K, Maeda H, Nedachi M, Hattori M, Iwasaki W, Sakata S. 2014. Methylocaldum marinum sp. nov., a thermotolerant, methane-oxidizing bacterium isolated from marine sediments, and emended description of the genus Methylocaldum. Int J Syst Evol Microbiol 64:3240–3246. doi: 10.1099/ijs.0.063503-0. [DOI] [PubMed] [Google Scholar]
- 44.Klappenbach JA, Dunbar JM, Schmidt TM. 2000. rRNA operon copy number reflects ecological strategies of bacteria. Appl Environ Microbiol 66:1328–1333. doi: 10.1128/aem.66.4.1328-1333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baani M, Liesack W. 2008. Two isozymes of particulate methane monooxygenase with different methane oxidation kinetics are found in Methylocystis sp. strain SC2. Proc Natl Acad Sci U S A 105:10203–10208. doi: 10.1073/pnas.0702643105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tavormina PL, Orphan VJ, Kalyuzhnaya MG, Jetten MS, Klotz MG. 2011. A novel family of functional operons encoding methane/ammonia monooxygenase‐related proteins in gammaproteobacterial methanotrophs. Environ Microbiol Rep 3:91–100. doi: 10.1111/j.1758-2229.2010.00192.x. [DOI] [PubMed] [Google Scholar]
- 47.Khalifa A, Lee CG, Ogiso T, Ueno C, Dianou D, Demachi T, Katayama A, Asakawa S. 2015. Methylomagnum ishizawai gen. nov., sp. nov., a mesophilic type I methanotroph isolated from rice rhizosphere. Int J Syst Evol Microbiol 65:3527–3534. doi: 10.1099/ijsem.0.000451. [DOI] [PubMed] [Google Scholar]
- 48.Reim A, Lüke C, Krause S, Pratscher J, Frenzel P. 2012. One millimetre makes the difference: high-resolution analysis of methane-oxidizing bacteria and their specific activity at the oxic-anoxic interface in a flooded paddy soil. ISME J 6:2128. doi: 10.1038/ismej.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez-Gomez NC, Vu HN, Skovran E. 2016. Lanthanide chemistry: from coordination in chemical complexes shaping our technology to coordination in enzymes shaping bacterial metabolism. Inorg Chem 55:10083–10089. doi: 10.1021/acs.inorgchem.6b00919. [DOI] [PubMed] [Google Scholar]
- 50.Pol A, Barends TR, Dietl A, Khadem AF, Eygensteyn J, Jetten MS, Op den Camp HJ. 2014. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ Microbiol 16:255–264. doi: 10.1111/1462-2920.12249. [DOI] [PubMed] [Google Scholar]
- 51.Keltjens JT, Pol A, Reimann J, Op den Camp HJ. 2014. PQQ-dependent methanol dehydrogenases: rare-earth elements make a difference. Appl Microbiol Biotechnol 98:6163–6183. doi: 10.1007/s00253-014-5766-8. [DOI] [PubMed] [Google Scholar]
- 52.Marison IW, Attwood MM. 1982. A possible alternative mechanism for the oxidation of formaldehyde to formate. Microbiology 128:1441–1446. doi: 10.1099/00221287-128-7-1441. [DOI] [Google Scholar]
- 53.Wilson SM, Gleisten MP, Donohue TJ. 2008. Identification of proteins involved in formaldehyde metabolism by Rhodobacter sphaeroides. Microbiology (Reading, Engl) 154:296. doi: 10.1099/mic.0.2007/011346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanson RS, Netrusov AI, Tsuji K. 1991. The obligate methanotrophic bacteria Methylococcus, Methylomonas, and Methylosinus, p 2350–2364. In Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (ed), The Prokaryotes, 2nd ed Springer-Verlag, New York, NY. [Google Scholar]
- 55.Attwood M, Quayle J. 1984. Formaldehyde as a central intermediary metabolite of methylotrophic metabolism, p 315–323. In Crawford RL, Hanson RS (ed), Microbial growth on C1 compounds. American Society for Microbiology, Washington, DC. [Google Scholar]
- 56.Zahn JA, Bergmann DJ, Boyd JM, Kunz RC, DiSpirito AA. 2001. Membrane-associated quinoprotein formaldehyde dehydrogenase from Methylococcus capsulatus Bath. J Bacteriol 183:6832–6840. doi: 10.1128/JB.183.23.6832-6840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mashhadi Z. 2010. Identification and characterization of the enzymes involved in biosynthesis of FAD and Tetrahydromethanopterin in Methanocaldococcus jannaschii. PhD dissertation. Virginia Tech, Blacksburg, VA. [Google Scholar]
- 58.Crowther GJ, Kosály G, Lidstrom ME. 2008. Formate as the main branch point for methylotrophic metabolism in Methylobacterium extorquens AM1. J Bacteriol 190:5057–5062. doi: 10.1128/JB.00228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marx CJ, Chistoserdova L, Lidstrom ME. 2003. Formaldehyde-detoxifying role of the tetrahydromethanopterin-linked pathway in Methylobacterium extorquens AM1. J Bacteriol 185:7160–7168. doi: 10.1128/jb.185.23.7160-7168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Studer A, Stupperich E, Vuilleumier S, Leisinger T. 2001. Chloromethane: tetrahydrofolate methyl transfer by two proteins from Methylobacterium chloromethanicum strain CM4. FEBS J 268:2931–2938. doi: 10.1046/j.1432-1327.2001.02182.x. [DOI] [PubMed] [Google Scholar]
- 61.Chistoserdova L, Laukel M, Portais J-C, Vorholt JA, Lidstrom ME. 2004. Multiple formate dehydrogenase enzymes in the facultative methylotroph Methylobacterium extorquens AM1 are dispensable for growth on methanol. J Bacteriol 186:22–28. doi: 10.1128/jb.186.1.22-28.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dalton H. 1979. The fortuitous oxidation and cometabolism of various carbon compounds by whole‐cell suspensions of Methylococcus capsulatus (Bath). FEMS Microbiol Lett 5:315–318. doi: 10.1111/j.1574-6968.1979.tb03329.x. [DOI] [Google Scholar]
- 63.Kletzin A, Adams MW. 1996. Tungsten in biological systems. FEMS Microbiol Rev 18:5–63. doi: 10.1016/0168-6445(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 64.Biegel E, Schmidt S, González JM, Müller V. 2011. Biochemistry, evolution and physiological function of the Rnf complex, a novel ion-motive electron transport complex in prokaryotes. Cell Mol Life Sci 68:613–634. doi: 10.1007/s00018-010-0555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hernandez ME, Beck DA, Lidstrom ME, Chistoserdova L. 2015. Oxygen availability is a major factor in determining the composition of microbial communities involved in methane oxidation. PeerJ 3:e801. doi: 10.7717/peerj.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han H, Hemp J, Pace LA, Ouyang H, Ganesan K, Roh JH, Daldal F, Blanke SR, Gennis RB. 2011. Adaptation of aerobic respiration to low O2 environments. Proc Natl Acad Sci U S A 108:14109–14114. doi: 10.1073/pnas.1018958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borisov VB, Gennis RB, Hemp J, Verkhovsky MI. 2011. The cytochrome bd respiratory oxygen reductases. Biochim Biophys Acta 1807:1398–1413. doi: 10.1016/j.bbabio.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anthony C. 1982. The biochemistry of methylotrophs. Academic Press, London, United Kingdom. [Google Scholar]
- 69.Newaz SS, Hersh LB. 1975. Reduced nicotinamide adenine dinucleotide-activated phosphoenolpyruvate carboxylase in Pseudomonas MA: potential regulation between carbon assimilation and energy production. J Bacteriol 124:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor SC, Dalton H, Dow CS. 1981. Ribulose-1,5-bisphosphate carboxylase/oxygenase and carbon assimilation in Methylococcus capsulatus (Bath). Microbiology 122:89–94. doi: 10.1099/00221287-122-1-89. [DOI] [Google Scholar]
- 71.Campbell MA, Nyerges G, Kozlowski JA, Poret-Peterson AT, Stein LY, Klotz MG. 2011. Model of the molecular basis for hydroxylamine oxidation and nitrous oxide production in methanotrophic bacteria. FEMS Microbiol Lett 322:82–89. doi: 10.1111/j.1574-6968.2011.02340.x. [DOI] [PubMed] [Google Scholar]
- 72.Kits KD, Klotz MG, Stein LY. 2015. Methane oxidation coupled to nitrate reduction under hypoxia by the Gammaproteobacterium Methylomonas denitrificans, sp. nov. type strain FJG1. Environ Microbiol 17:3219–3232. doi: 10.1111/1462-2920.12772. [DOI] [PubMed] [Google Scholar]
- 73.Ho A, Lüke C, Cao Z, Frenzel P. 2011. Ageing well: methane oxidation and methane oxidizing bacteria along a chronosequence of 2000 years. Environ Microbiol Reports 3:738–743. doi: 10.1111/j.1758-2229.2011.00292.x. [DOI] [PubMed] [Google Scholar]
- 74.Murase J, Frenzel P. 2007. A methane‐driven microbial food web in a wetland rice soil. Environ Microbiol 9:3025–3034. doi: 10.1111/j.1462-2920.2007.01414.x. [DOI] [PubMed] [Google Scholar]
- 75.de Jong AEE, In ’t Zandt MH, Meisel OH, Jetten MSM, Dean JF, Rasigraf O, Welte CU. 2018. Increases in temperature and nutrient availability positively affect methane‐cycling microorganisms in Arctic thermokarst lake sediments. Environ Microbiol 20:4314–4327. doi: 10.1111/1462-2920.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Illumina. 2019. MiSeq System denature and dilute libraries guide. Illumina, San Diego, CA. [Google Scholar]
- 77.Albertsen M, Hugenholtz P, Skarshewski A, Nielsen KL, Tyson GW, Nielsen PH. 2013. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat Biotechnol 31:533. doi: 10.1038/nbt.2579. [DOI] [PubMed] [Google Scholar]
- 78.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoefman S, van der Ha D, Iguchi H, Yurimoto H, Sakai Y, Boon N, Vandamme P, Heylen K, De Vos P. 2014. Methyloparacoccus murrellii gen. nov., sp. nov., a methanotroph isolated from pond water. Int J Syst Evol Microbiol 64:2100–2107. doi: 10.1099/ijs.0.057760-0. [DOI] [PubMed] [Google Scholar]
- 81.Geymonat E, Ferrando L, Tarlera S. 2011. Methylogaea oryzae gen. nov., sp. nov., a mesophilic methanotroph isolated from a rice paddy field. Int J Syst Evol Microbiol 61:2568–2572. doi: 10.1099/ijs.0.028274-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth dynamics of strain C50C1 in 5-fold-diluted ammonium-containing (circles) or nitrogen-free (squares) mineral salts medium (AMS and MS, respectively) under atmospheric (open symbols) and low (solid symbols) O2 levels. Optical density was measured at 600 nm. Arrows represent O2 replenishment. Download FIG S1, PDF file, 0.04 MB (335.5KB, pdf) .
Copyright © 2019 Ghashghavi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PLFA profiles of type I and verrucomicrobial MOB compared by nonmetric multiscale dimensional scaling (NMDS) of PLFA profiles of methanotroph cultures (expressed as percentages of total PLFA content). The two-dimensional distances between samples in the NMDS plot show the relative similarity between samples. The closest matching PLFA profiles are indicated with a minimum spanning tree analysis displaying the shortest distance (i.e., similarity) to connect all PLFA profiles, which results in relating a profile to its nearest neighbor for every sample. The analyses were carried out with the software PAST. Black dots, type Ia; blue squares, type Ib; red triangles, Methylothermaceae (type Ic); green dots, Methylacidiphilaceae (type III). Download FIG S2, PDF file, 0.3 MB (39KB, pdf) .
Copyright © 2019 Ghashghavi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Compositions of AMS, NMS, and MS media (as described by Whittenbury et al. [16]) and trace element solutions. Download Table S1, DOCX file, 0.02 MB (15.7KB, docx) .
Copyright © 2019 Ghashghavi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Substrate utilization pattern of strain C50C1. Download Table S2, DOCX file, 0.02 MB (15.2KB, docx) .
Copyright © 2019 Ghashghavi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Phospholipid fatty acid (PLFA) profiles of strain C50C1. Download Table S3, DOCX file, 0.02 MB (16.5KB, docx) .
Copyright © 2019 Ghashghavi et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
The high-quality draft genome of strain C50C1 is available at NCBI under BioProject accession number PRJNA361434.