Figure 5.
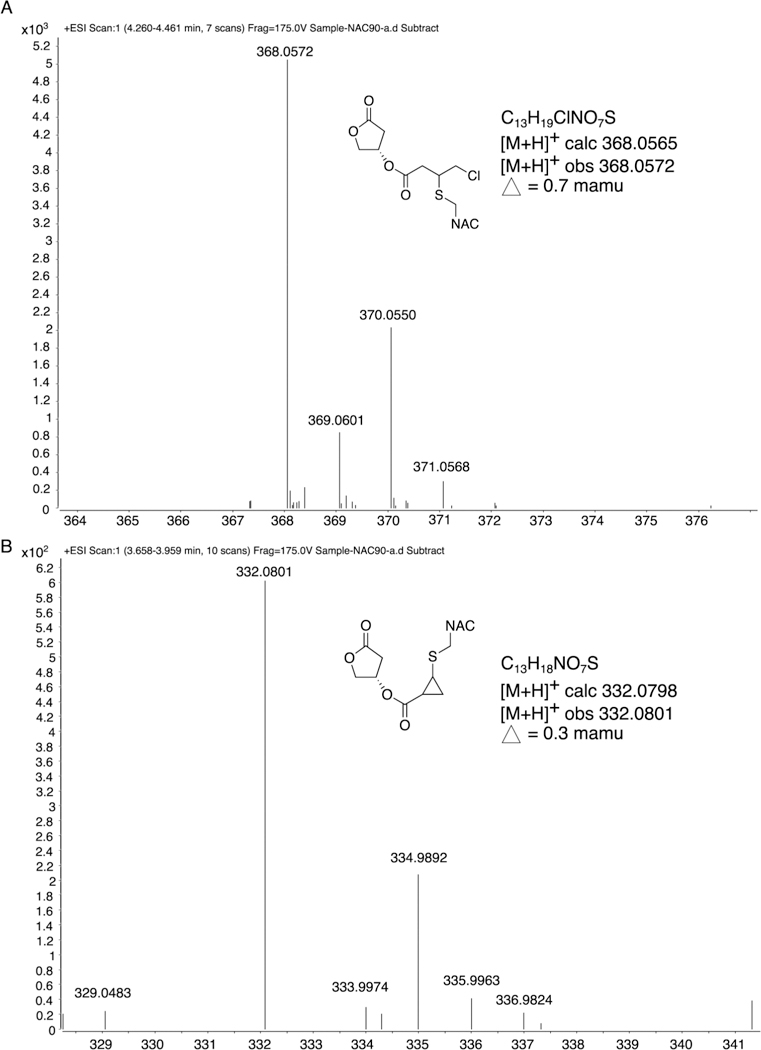
Reaction of honaucin A with the cysteine-bearing small molecule N-acetylcysteine (NAC) through 1,4-conjugate addition to produce two products. The later eluting peak was centered at 4.3 min (A) and had an [M + H]+ m/z 368.0572 (C13H19ClNO7S) for the SNAC addition product, whereas the earlier eluting peak was centered at 3.8 min (B) and showed an [M + H]+ m/z 332.0801 (C13H18NO7S) for the SNAC addition plus loss of HCl to produce the proposed cyclopropyl ring compound. This indicates that honaucin A may stabilize Nrf2 by engaging sulfhydryl residues of the repressor protein Keap1. See also Figures S1 and S2.
