Extended Data Figure 1.
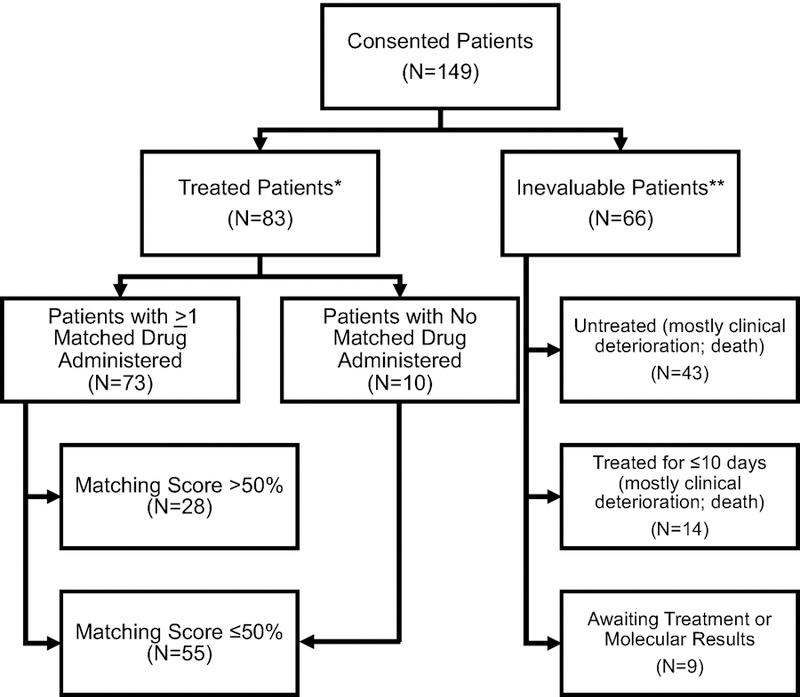
Consolidated Standards of Reporting Trials (CONSORT) diagram, which includes the 149 patients that consented to I-PREDICT.
* Treated evaluable patients includes patients who received >10 d of treatment for drugs given on a daily basis (generally drugs given by mouth) or at least two doses of a drug normally given every two weeks or more frequently (the latter generally being intravenous drugs). Only patients whose treatment was reviewed and validated by data analysis lockdown are included.
** One patient had inadequate tissue for NGS and declined biopsy; he was later reenrolled after he agreed to undergo biopsy.
Note: One treated patient who initially was believed to have prior therapy was found, after data lockdown analysis, to have not received the prior regimen.
